Answered step by step
Verified Expert Solution
Question
1 Approved Answer
help urgently Consider a gas contained in a cylinder that initially undergoes Isothermal expansion form a pressure of 4.5104mmHg to 52bar, before undergoing adiabatic expansion
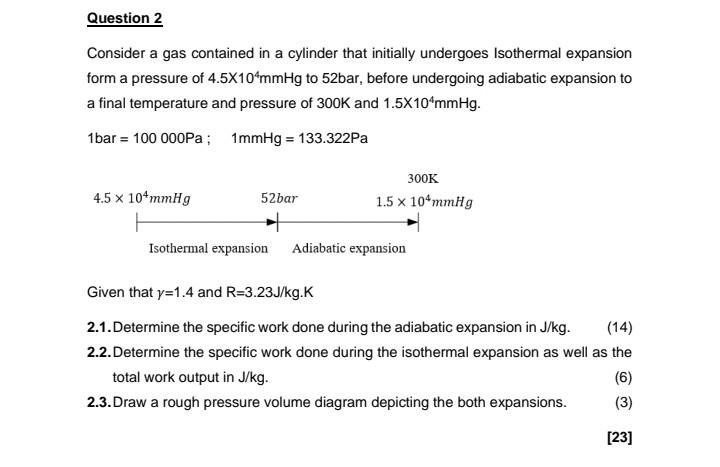
help urgently
Consider a gas contained in a cylinder that initially undergoes Isothermal expansion form a pressure of 4.5104mmHg to 52bar, before undergoing adiabatic expansion to a final temperature and pressure of 300K and 1.5104mmHg. 1bar=100000Pa;1mmHg=133.322Pa Given that =1.4 and R=3.23J/kg.K 2.1. Determine the specific work done during the adiabatic expansion in J/kg. 2.2. Determine the specific work done during the isothermal expansion as well as the total work output in J/kg. (6) 2.3. Draw a rough pressure volume diagram depicting the both expansions. (3)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


