Answered step by step
Verified Expert Solution
Question
1 Approved Answer
help with 7-9 please Consider the following hypothetical acid-base titration where A represents the acid and B represents the base. The products of this reaction
help with 7-9 please 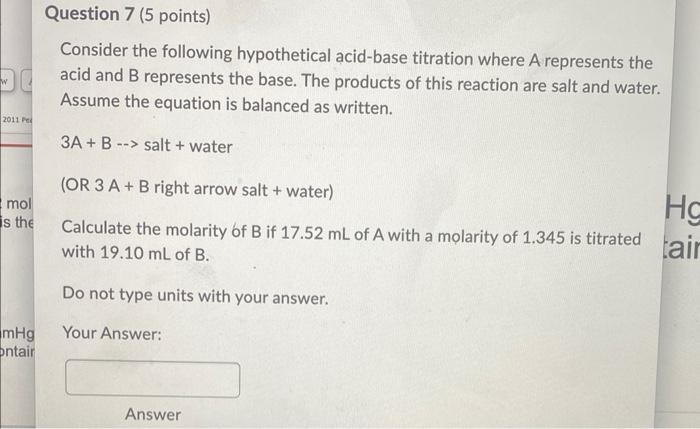


Consider the following hypothetical acid-base titration where A represents the acid and B represents the base. The products of this reaction are salt and water. Assume the equation is balanced as written. 3A+B salt + water (OR 3A+B right arrow salt + water) Calculate the molarity of B if 17.52mL of A with a molarity of 1.345 is titrated with 19.10mL of B. Do not type units with your answer. Your Answer: Consider the following hypothetical acid-base titration where A represents the acid and B represents the base. The products of this reaction are salt and water Assume the equation is balanced as written. 2A+Bsalt+water (OR 2A+B right arrow salt + water) Two burets are setup with one containing acid, A, and the other containing base, B. The initial volume of buret A is 1.56mL and the initial volume of buret B is 1.68mL. Acid from buret A is added to a flask until the final volume of buret A is 13.98mL. Base from buret B is added to the flask until the titration reaches the endpoint. The final volume of buret B reads 18.22mL. Calculate the molarity of the acid, A, if the base has a molarity of 0.683 molar. You may want to create a data table to help you organize your work. Do not type units with your answer. Your Answer: Consider the following hypothetical chemical equation 2A+3BA2B3 (OR 2A+3B right arrow A2B3 ) Calculate the mass in grams of A2B3 produced from the reaction of 141.70mL of 1.08MB with an excess amount of A. The atomic weight of A is 30grams/mole The atomic weight of B is 50grams/mole Your 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


