Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Help with these please. It is 16 page assignment and this section I can't get right. Equilibrium 1: Cu(OH)2(s)Cu2+(aq)+2OH(aq)K1=KspofCu(OH)2=[Cu2+][OH]2=2.21020 Equilibrium 2: 2H3O+(aq)+2OH(aq)4H2O(l) K2=[H3O+]2[OH]21 Net Equilibrium:
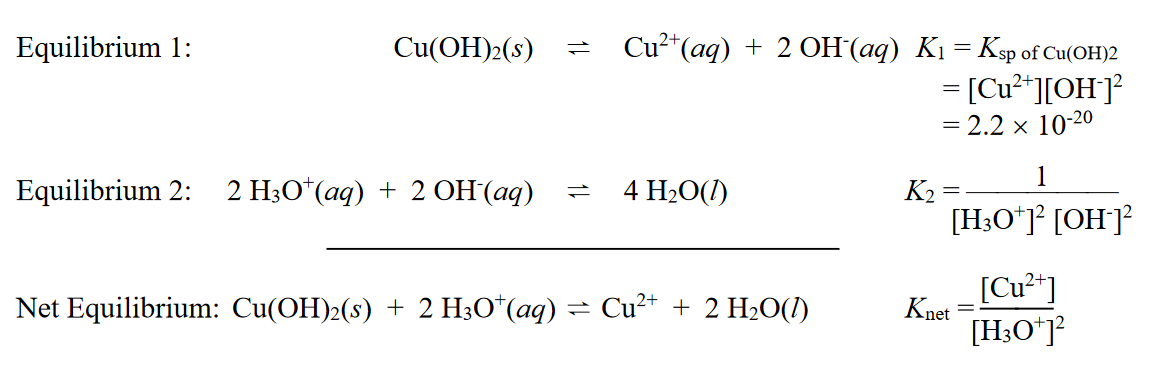
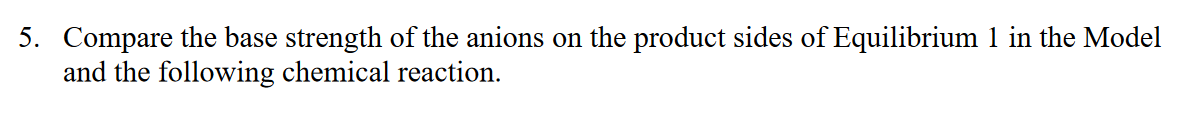
Help with these please. It is 16 page assignment and this section I can't get right.
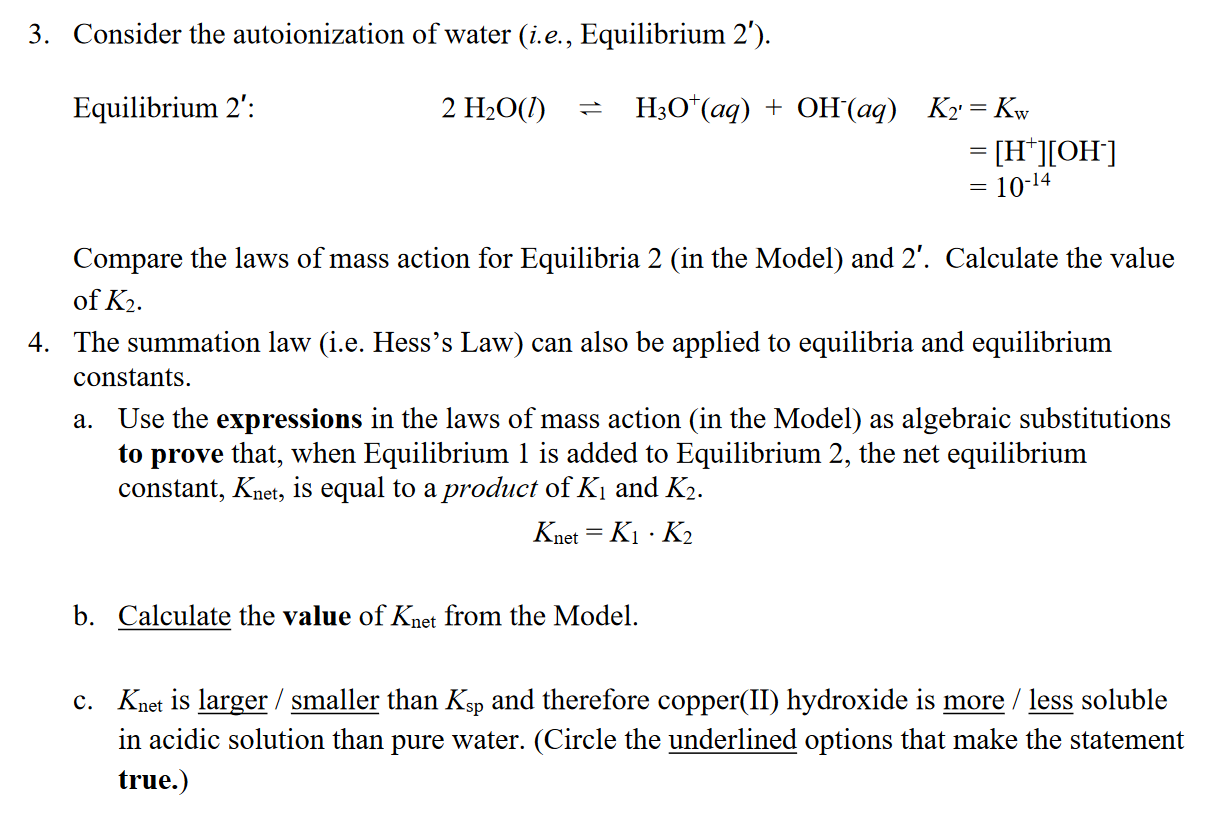
Equilibrium 1: Cu(OH)2(s)Cu2+(aq)+2OH(aq)K1=KspofCu(OH)2=[Cu2+][OH]2=2.21020 Equilibrium 2: 2H3O+(aq)+2OH(aq)4H2O(l) K2=[H3O+]2[OH]21 Net Equilibrium: Cu(OH)2(s)+2H3O+(aq)Cu2++2H2O(l)Knet=[H3O+]2[Cu2+] 3. Consider the autoionization of water (i.e., Equilibrium 2 ). Equilibrium 2': 2H2O(l)H3O+(aq)+OH(aq)K2=Kw=[H+][OH]=1014 Compare the laws of mass action for Equilibria 2 (in the Model) and 2'. Calculate the value of K2. 4. The summation law (i.e. Hess's Law) can also be applied to equilibria and equilibrium constants. a. Use the expressions in the laws of mass action (in the Model) as algebraic substitutions to prove that, when Equilibrium 1 is added to Equilibrium 2, the net equilibrium constant, Knet, is equal to a product of K1 and K2. Knet=K1K2 b. Calculate the value of Knet from the Model. c. Knet is larger / smaller than Ksp and therefore copper(II) hydroxide is more / less soluble in acidic solution than pure water. (Circle the underlined options that make the statement true.) 5. Compare the base strength of the anions on the product sides of Equilibrium 1 in the Model and the following chemical reactionStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


