Answered step by step
Verified Expert Solution
Question
1 Approved Answer
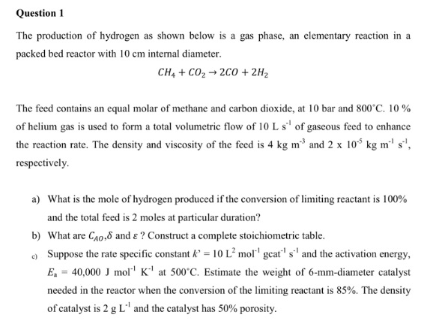
helpQuestion 1 The production of hydrogen as shown below is a gas phase, an elementary reaction in a packed bed reactor with 1 0 c
helpQuestion
The production of hydrogen as shown below is a gas phase, an elementary reaction in a
packed bed reactor with internal diameter.
The feed contains an equal molar of methane and carbon dioxide, at bar and
of helium gas is used to form a total volumetric flow of of gaseous feed to enhance
the reaction rate. The density and viscosity of the feed is and
respectively.
a What is the mole of hydrogen produced if the conversion of limiting reactant is
and the total feed is moles at particular duration?
b What are and Construct a complete stoichiometric table.
c Suppose the rate specific constant and the activation energy,
at Estimate the weight of mmdiameter catalyst
needed in the reactor when the conversion of the limiting reactant is The density
of catalyst is and the catalyst has porosity.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


