Question: Hey i need these done ASAP please. D2L Course Project - CTS S X D2L Assignment - Module x P30Module8Assignme X P30Module 7 Assignme X
Hey i need these done ASAP please.
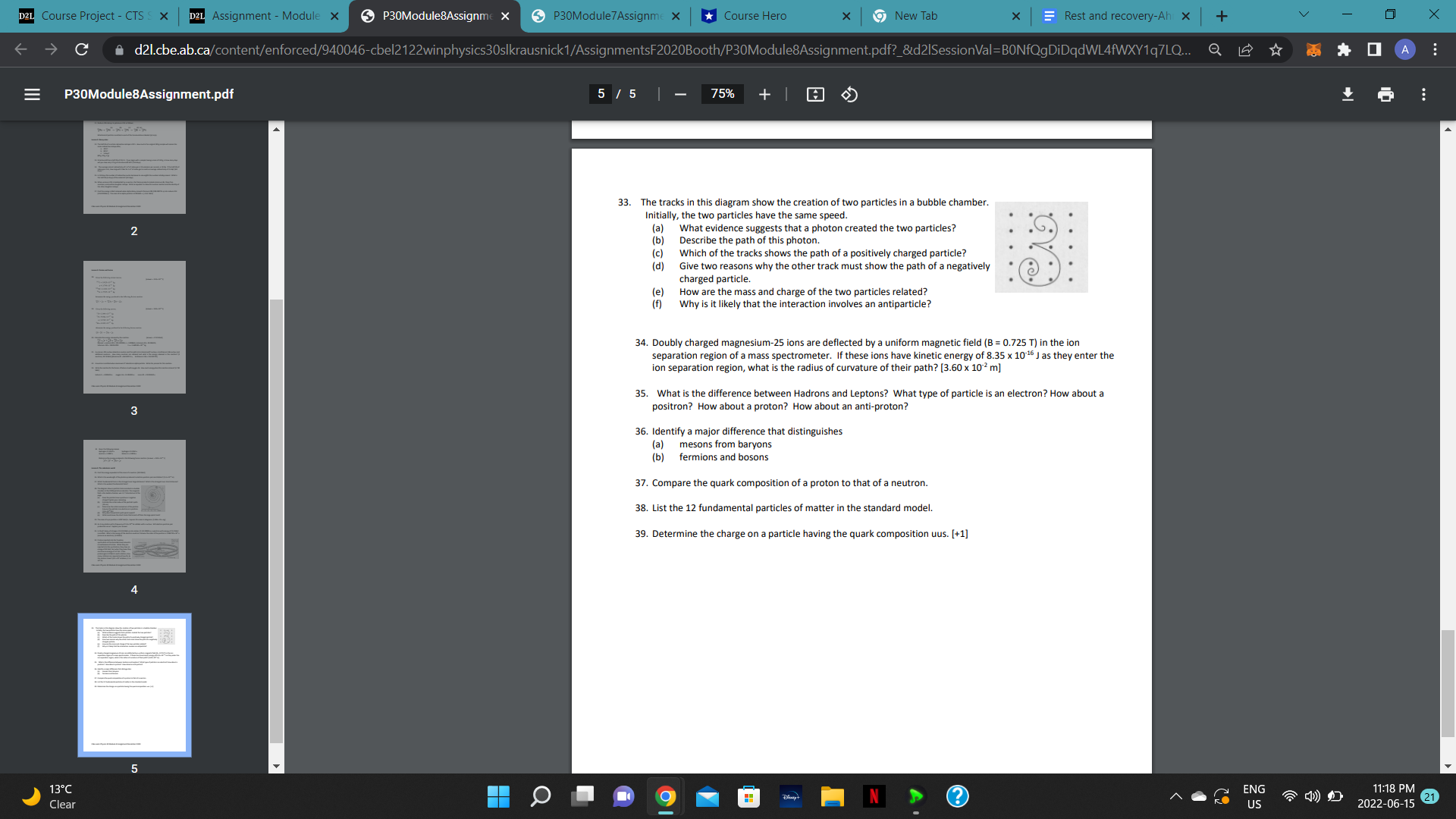
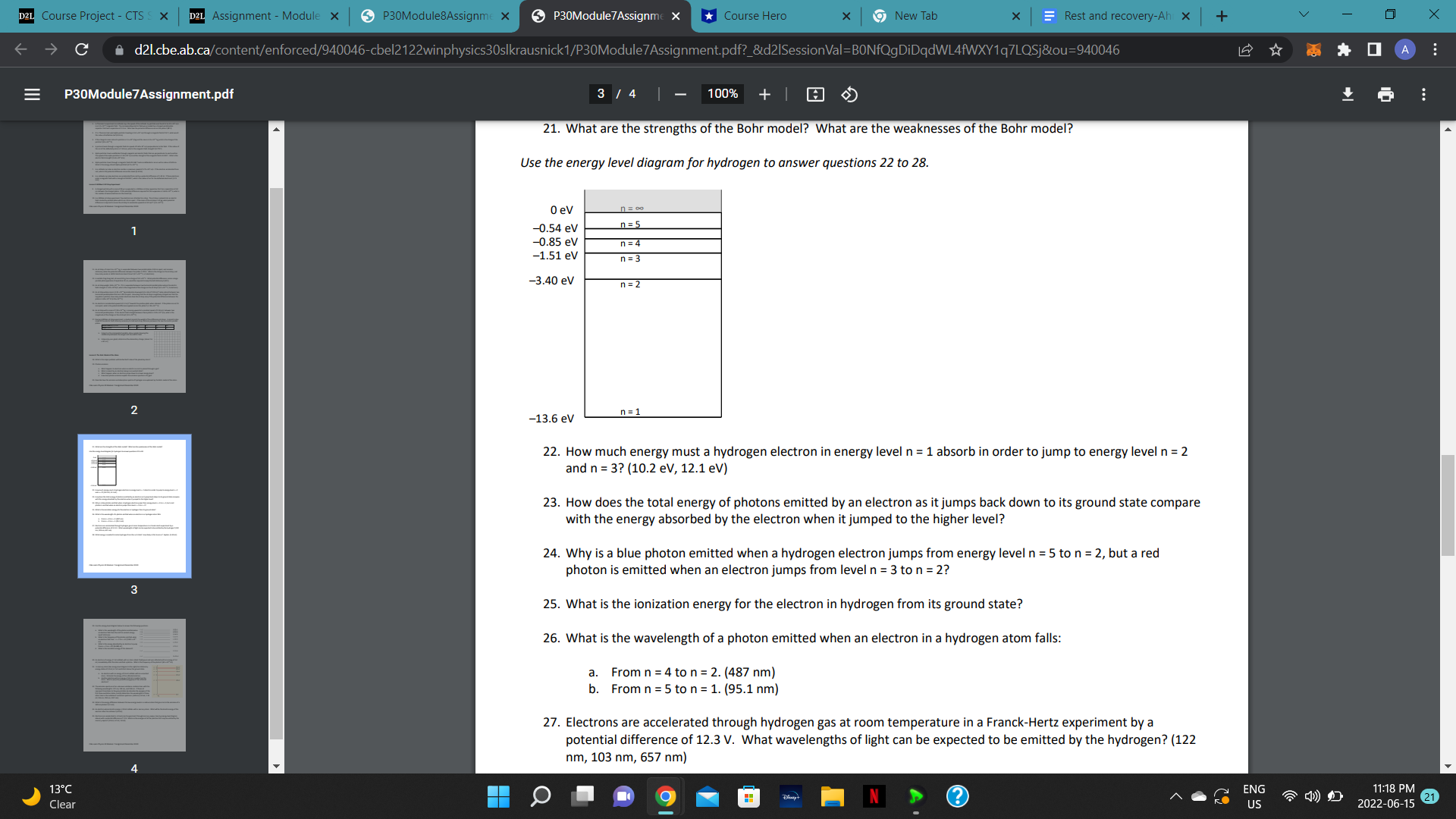
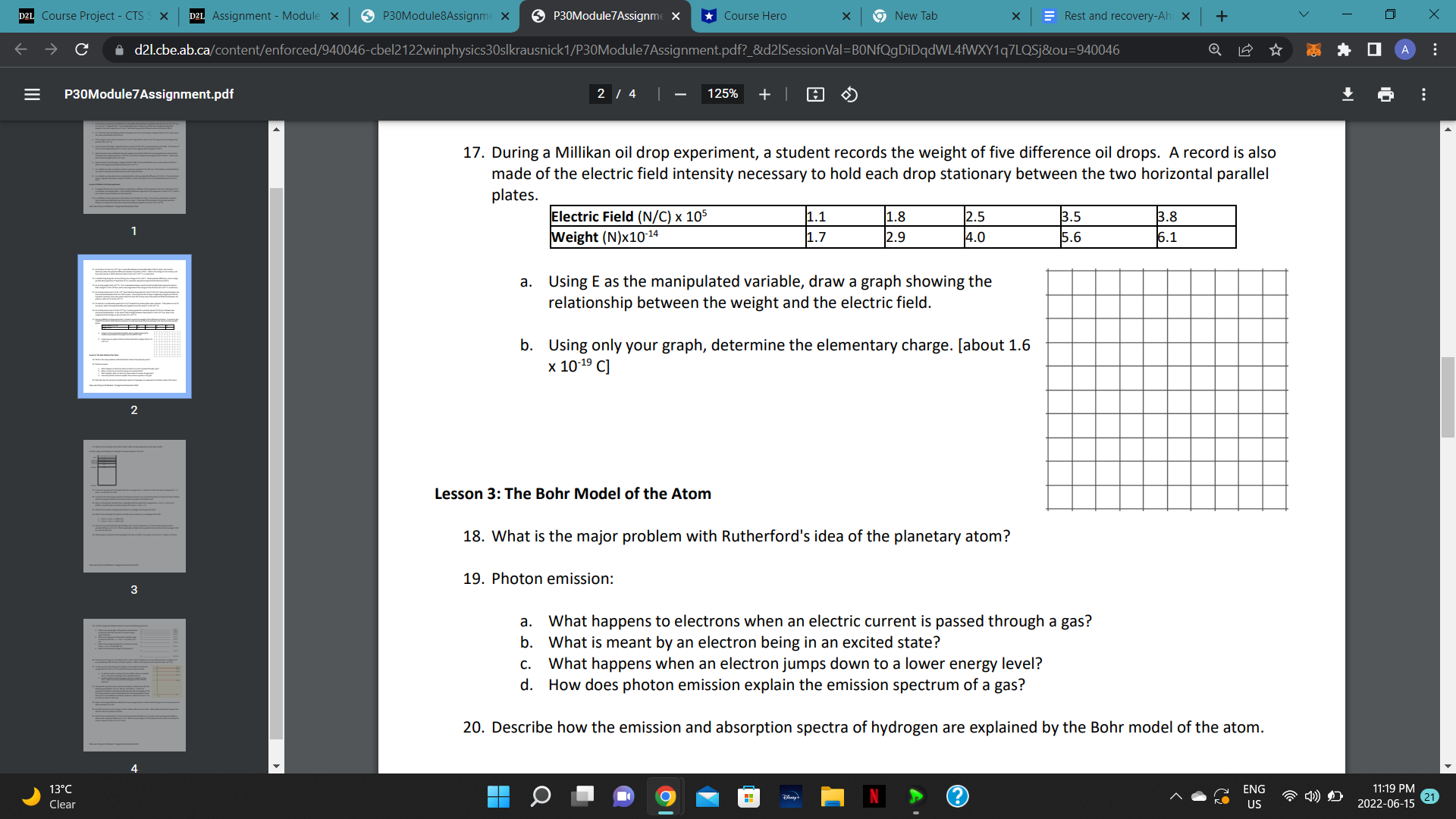
D2L Course Project - CTS S X D2L Assignment - Module x P30Module8Assignme X P30Module 7 Assignme X Course Hero x 9New Tab X Rest and recovery-Ah X + V X C d21.cbe.ab.ca/content/enforced/940046-cbel2122winphysics30slkrausnick1/AssignmentsF2020Booth/P30Module8Assignment.pdf?_&d21SessionVal=BONfQgDiDqdWL4fWXY1q7LQ... Q 0 A E P30Module8Assignment.pdf 5 / 5 | 75% + 33. The tracks in this diagram show the creation of two particles in a bubble chamber. Initially, the two particles have the same speed. 2 (a) What evidence suggests that a photon created the two particles? (b) Describe the path of this photon. (c) Which of the tracks shows the path of a positively charged particle? (d) Give two reasons why the other track must show the path of a negatively charged particle (e) How are the mass and charge of the two particles related? Why is it likely that the interaction involves an antiparticle? 34. Doubly charged magnesium-25 ions are deflected by a uniform magnetic field (B = 0.725 T) in the ion separation region of a mass spectrometer. If these ions have kinetic energy of 8.35 x 10-16 J as they enter the ion separation region, what is the radius of curvature of their path? [3.60 x 102 m] 35. What is the difference between Hadrons and Leptons? What type of particle is an electron? How about a 3 positron? How about a proton? How about an anti-proton? 36. Identify a major difference that distinguishes (a) mesons from baryons b) fermions and bosons 37. Compare the quark composition of a proton to that of a neutron. 38. List the 12 fundamental particles of matter in the standard model. 39. Determine the charge on a particle having the quark composition uus. [+1] 5 13 C ENG 11:18 PM Clear US 2022-06-15 21D2L Course Project - CTS S X D2L Assignment - Module x P30Module8Assignme X P30Module 7 Assignme X *Course Hero * 9 New Tab X Rest and recovery-Ah x + V X C A d21.cbe.ab.ca/content/enforced/940046-cbel2122winphysics30slkrausnick 1/P30Module7Assignment.pdf?_&d21SessionVal=BONfQgDiDqdWL4fWXY1q7LQSjou=940046 0 A E P30Module7 Assignment.pdf 3 / 4 100% + 21. What are the strengths of the Bohr model? What are the weaknesses of the Bohr model? Use the energy level diagram for hydrogen to answer questions 22 to 28. 0 ev -0.54 ev n = 5 -0.85 ev n = 4 -1.51 ev n = 3 -3.40 ev n = 2 2 -13.6 ev n = 1 22. How much energy must a hydrogen electron in energy level n = 1 absorb in order to jump to energy level n = 2 and n = 3? (10.2 ev, 12.1 eV) 23. How does the total energy of photons emitted by an electron as it jumps back down to its ground state compare with the energy absorbed by the electron when it jumped to the higher level? 24. Why is a blue photon emitted when a hydrogen electron jumps from energy level n = 5 to n = 2, but a red photon is emitted when an electron jumps from level n = 3 to n = 2? 3 25. What is the ionization energy for the electron in hydrogen from its ground state? 26. What is the wavelength of a photon emitted when an electron in a hydrogen atom falls: a. From n = 4 to n = 2. (487 nm) b. From n = 5 to n = 1. (95.1 nm) 27. Electrons are accelerated through hydrogen gas at room temperature in a Franck-Hertz experiment by a potential difference of 12.3 V. What wavelengths of light can be expected to be emitted by the hydrogen? (122 nm, 103 nm, 657 nm) ENG 11:18 PM Clear O US 2022-06-15 21C le cl)e.al3.i:a P30Mudule7Assignmenl.pdf g F'BDModule'fAsslgm 17. During a Millikan oil drop experiment. a student records the weight of five difference oil drops. A record is also made of the electric field intensity necessary to hold each drop stationary between the two horizontal parallel plates. Electric Field (WC) )4 105 1.1 1.8 .8 2.5 _ Weight (N)x10'\" 1.7 2.9 |4.o |5.e |s.1 Using E as the manipulated variable, draw a graph showingthe relationship between the weight and the electric field. Using only your graph. determine the elementary charge. [about 1.6 x 10'19 C] Lesson 3: The Bohr Model ofthe Atom 18. What is the major problem with Rutherford's idea of the planetary atom? 19. Photon emission: What happens to electrons when an electric current is passed through a gas? What is meant by an electron being in an excited state? What happens when an electron jumps down to a lower energy level? How does photon emission explain the emission spectrum of a gas? 20. Describe how the emission and absorption spectra of hydrogen are explained by the Bohr model ofthe atom
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


