Hi.. Answer all questions
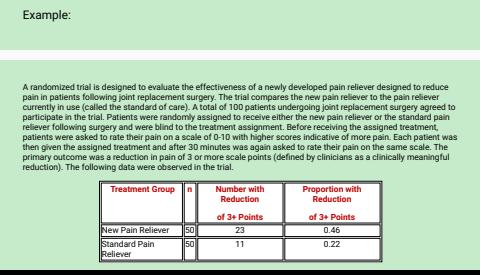
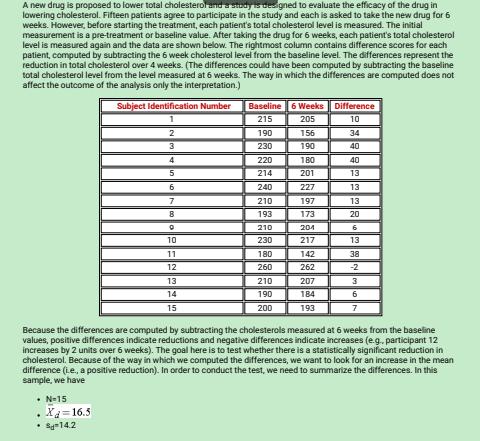
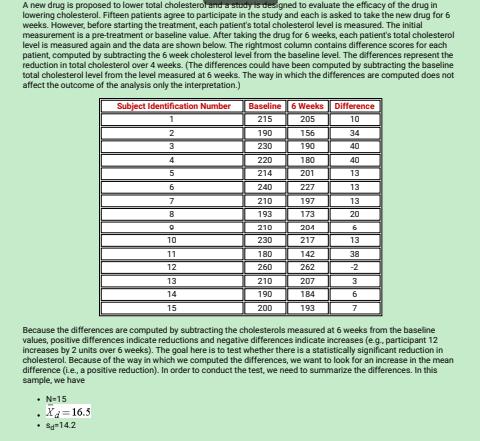
Example: A randomized trial is designed to eve bate the effectiveness of a newly developed pain reliever designed to reduce pain in patients following joint replacement surgery. The trial compares the new pain reliever to the pain rebever currently in use (called the standard of care). A total of 100 patients undergoing joint replacement surgery agreed to participate in the trial. Patients were randomly assigned to receive either the new pain reliever of the standard pain reliever following surgery and were blind to the treatment assignment. Before receiving the assigned treatment, patients were asked to rate their pain on a scale of 0-10 with higher scores indicative of more pain. Each patient was then given the assigned treatment and after 30 minutes was again asked to rate their pain on the same scale. The primary outcome was a reduction in pain of 3 or more scale points (defined by clinicians as a clinically meaningful reduction). The following data were observed in the trial. Treatment Group Number with Proportion with Reduction Reduction of 3+ Points of 3+ Points New Pain Reliever 50 23 0.46 Standard Pain 50 11 0.22 RelieverA new drug is proposed to lower total cholesterol and a designed to evaluate the efficacy of the drug in lowering cholesterol. Fifteen patients agree to participate in the study and each is asked to take the new drug for 6 weeks. However, before starting the treatment, each patient's total cholesterol level is measured. The initial measurement is a pre-treatment or baseline value. After taking the drug for 6 weeks, each patient's total cholesterol level is measured again and the data are shown below. The rightmost column contains difference scores for each patient, computed by subtracting the 6 week cholesterol level from the baseline level. The differences represent the reduction in total cholesterol over 4 weeks. (The differences could have been computed by subtracting the baseline total cholesterol level from the level measured at 6 weeks. The way in which the differences are computed does not affect the outcome of the analysis only the interpretation.) Subject Identification Number Baseline 6 Weeks Difference 215 205 10 190 156 34 230 061 40 4 220 180 40 5 214 201 13 240 227 12 210 197 13 193 173 20 210 204 6 10 230 217 13 17 180 142 38 12 260 262 -2 13 210 207 14 190 184 6 15 200 193 7 Because the differences are computed by subtracting the cholesterols measured at 6 weeks from the baseline values, positive differences indicate reductions and negative differences indicate increases (e.g, participant 12 Increases by 2 units over 6 weeks). The goal here is to test whether there is a statistically significant reduction in cholesterol. Because of the way in which we computed the differences, we want to look for an increase in the mean difference (Le., a positive reduction). In order to conduct the test, we need to summarize the differences. In this sample, we have . N-15 X=16.5 By 14.2