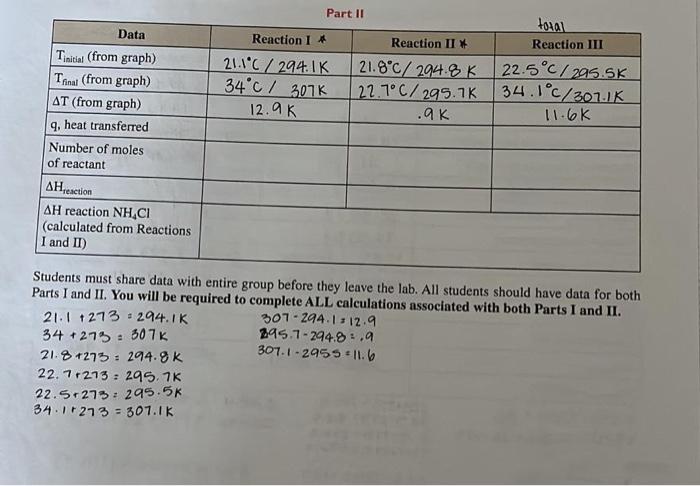
Hi can you please check if my calcuations are correct for part 1 and for part 2 can you assit me with the remaining. I am lost. Thank you!!
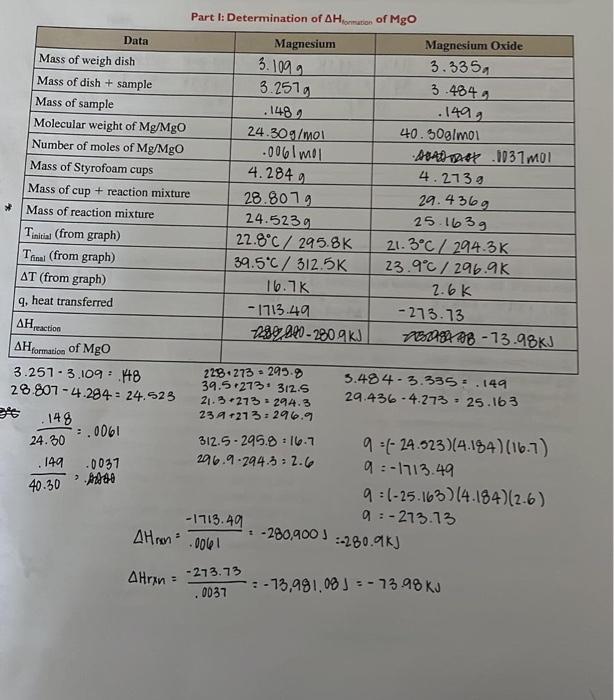
Part I: Determination of Hlonmution of MgO symbol AH, called the enthalpy change. Different enthalpy changes for dilferent fypes of reactions fsuch as formation and reaction) can be determined. Please feler to your text book for these For this experiment, acing. Vernier LnhQoest 2 data collection interface a is exothermic. When q is calculated, then the value will be negative. The negattive sign anmply indicatesthe direction of beat flow, not the quantify. If T is negative, heat was abtorbed from the sotution, the reaction is ner mole of rubstance, which can be demonstrated by the following equation: QraH=qri/molmatatane To measure the enthalpy change (AH) of a reaction directly is often difficult and sometimes impossible. depending on the reaction time, the pathway, of the lack of product stahility. The enthalpy change of that reaction can however be determined indirectly from enthalpy changes for other reactions that can be measured. We. can ealenlate the enthalpy change by using Hes." Iaw, which states that if a reaction is the sum of rwo or more reactions. the enthalpy for the overall process is the sum of the emhalpy valoes of those reactions (Kotz, refer to 5.7 in your textbook). According to Hess's law, yot can measure the enthalpy of two reactions and add them to get the enthalpy of the total reaction, as in the following example. Note: compounds found on both sides of the reaction cancel each other out. C(s)+1/2O2(g)CO(g)H1=110.5kJ/molCOwi+1/2O2wCO2wAH2=283.0kJ/molCisi+O2iCO2wHT=393.5kJ/mol In today's experiment, you will be determining the enthalpies of two separate reactions. In Part 1 of this experiment you will be determining the Hfommon of MgO and in Part II of this experiment you will be determining the AHnextian of NH4Cl. Part I For Part I, you will work in groups of two or three to determine the enthalpy change for the reactions of Mg and MgO with HCl to solve for Hformation of MgO. You will start with Mg metal. One partner will measure the enthalpy change when Mg is added to 1.0MHCl and measure the heat evolved or absorbed with a coffeecup calorimeter (refer to 5.6 in your text to review calorimetry). The other partner will measure the enthalpy change that occurs when a measured amount of the MgO is added to 1.0MHCl Once you determine the enthalpies of the two reactions, you can set up and solve the following reactions and apply Hess's law: (Note: equations are already balanced.) Mg(0)+2HCl(aq)MgCl2(aq)+H2ia)H1=?kJ/molMgO(a)+2HCl(aq)MgCl2(aq)+H2O(1)H2=?kJ/mol To make these equations add up to the formation reaction of MgO, you will also need to include a basic equation with a published enthalpy. That equation is: H2id+H2O2(g)H2O(n) solve for AHformanion of MgO. Part II In Part II of today's experiment, you will also be working in groups of two or three to perform two tasks. For Task 1, you will measure the temperature change of two reactions: HCl(aq)+NaOH(aq)NaCl+H2O(5)H1=?kJ/molNaOH(aq)+NH4Cl(aq)NH3(aq)+H2O(0)+NaCl(aq)H2=?kJ/mol One partner will measure the enthalpy change for the first reaction of 2.0MNaOH and 2.0MHCl, while the other partner measures the enthalpy change for the second reaction between 2.0M NaOH and 2.0M NH4Cl. Using Hess's law, you can manipulate these equations to determine the HreactionofNH4Cl from NH3(aq) and HCl(aq) For Task 2, both students will work together to directly measure the enthalpy change for the reaction between NH3(av) and HCl. NH(aq)+HCl(aq)NH4Cl(aq)H3=?kJ/mol This reaction, Reaction III, allows a direct, one-step reaction to determine the HreactionOfNH4Cl.This third reaction is performed to compare the results from determining the Hrection of NH4Clfrombothdirect and indirect means. Note: The specific heat capacity Part I 1. Calculate the mass of your metal by difference. 2. Calculate the molecular weight and then the number of moles of your metal and metal oxide. 3. Calculate T : T=TfinalTintial 4. Calculate the mass of the reaction mixture (Solid added to a solution) by difference. 5. Calculate q, the heat transferred with the metal and metal oxide. 6. Calculate Hraction per mole of Mg and MgO. 7. Utilizing the three equations provided and your knowledge of Hess's law, show how they can be manipulated to determine the equation and final value for HformationofMgO. Show balanced equations, cancelled out compounds, and enthalpies of all reactions as seen in the example in this lab. Part II 1. Calculate T:T=TfinalTinitial 2. Calculate the heat transferred q for each of the three reactions. Use 1.03g/mL for the density of all solutions. 3. Calculate the number of moles of each reactant from the provided concentration (for Reaction 1, you solving for moles of HCl; for Reaction II, you are solving for moles of NH4Cl; and for Reaction III, y are solving for moles of NH3 ). 4. Calculate Hreaction per mole of HCl,NH4Cl, and NH3, in kJ/mol. 5. Using the data collected for Reactions I and II, apply Hess's law to determine HforNH4Cl. 6. Using the equations provided from Reactions I and II, and your knowledge of Hess's law, show hov they can be manipulated to determine the equation and final value for Hreaction of NH4Cl. Show bal anced equations, cancelled-out compounds, and enthalpies of all reactions as seen in the example this lab. Students must share data with entire group before they leave the lab. All students should have data for both Parts I and II. You will be required to complete ALL calculations associated with both Parts I and II. 34+273=307k21.8+273=294.8k295.7294.8=.9307.12955=11.622.7+273=295.7K22.5+273=295.5k34.1+273=307.1K