Answered step by step
Verified Expert Solution
Question
1 Approved Answer
hi, i already given to you all information with answer also. just u need to show me the mass balance steps for solve this cstr
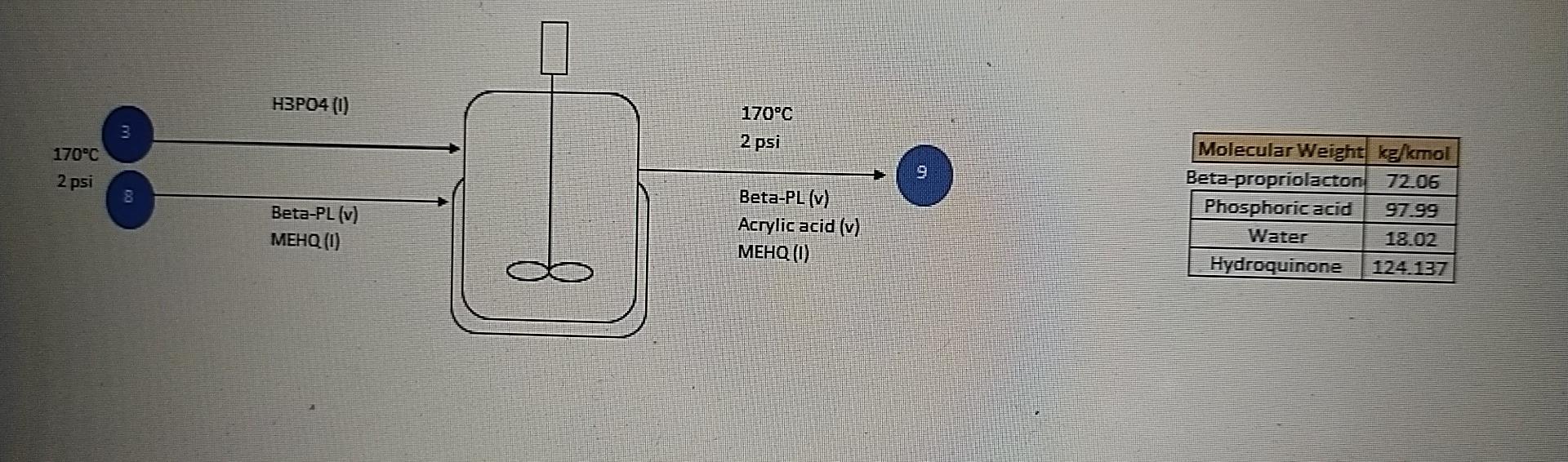
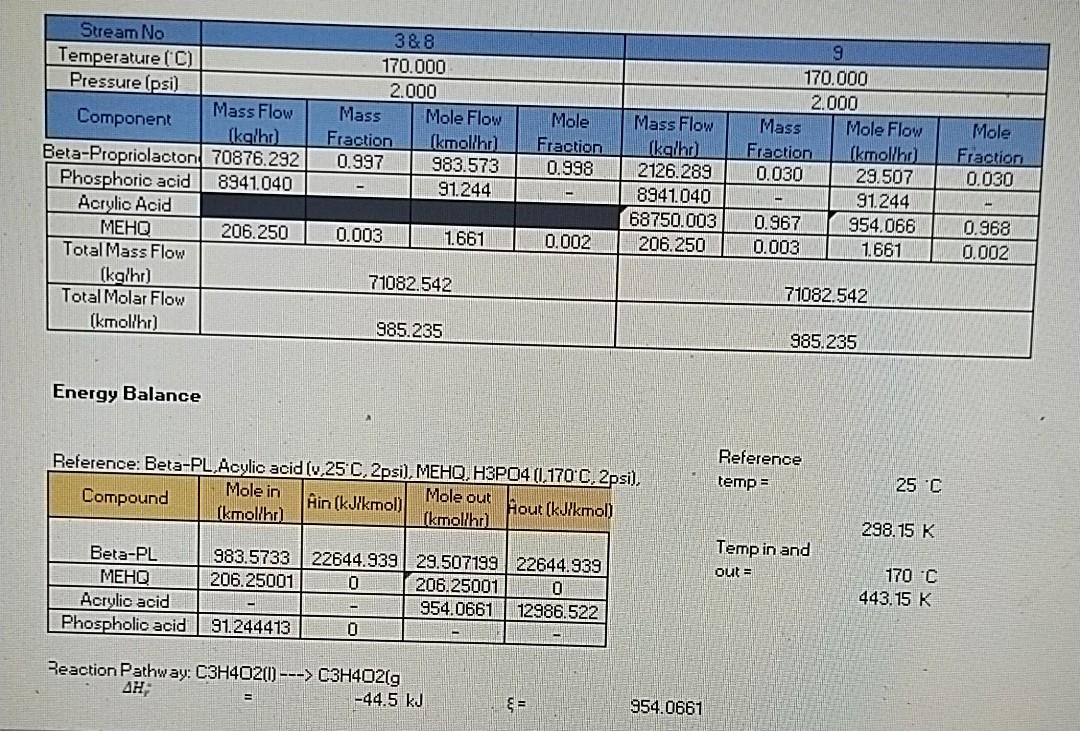
hi, i already given to you all information with answer also. just u need to show me the mass balance steps for solve this cstr unit operation
Do the mass balance step by step, and get the same answer that i provided in picture box .
H3PO4 (0) mry 170C 2 psi 170C 2 psi Beta-PL (v) MEHQ (0) Beta-PL (v) Acrylic acid (v) MEHQ (0) Molecular Weight kg/kmol Beta-propriolacton 72.06 Phosphoric acid 97.99 Water 18.02 Hydroquinone 124.137 Stream No Temperature (C) Pressure (psi) Mass Flow Component (kalhr) Beta-Propriolacton 70876.292 Phosphoric acid 8941.040 Acrylic Acid MEHO 206.250 Total Mass Flow (kalhr) Total Molar Flow (kmol/hr) 388 170.000 2.000 Mass Mole Flow Fraction (kmollhr) 0.997 983.573 91.244 Mole Fraction 0.998 Mass Flow (kalho) 2126.289 8941.040 68750.003 206.250 9 170.000 2.000 Mass Mole Flow Fraction Ikmollho) 0.030 29.507 91244 0.967 954.066 0.003 Mole Fraction 0.030 0.003 1.661 0.002 1.661 0.968 0.002 71082.542 71082.542 985.235 985.235 Energy Balance Reference: Beta-PL Acylic acid (v,25 C, 2psi), MEHQ, H3PO4 (1,170-C, 2psi), Mole in Mole out Compound Hin (kJ/kmol) Hout (kJ/kmol) (kmollho) [kmollhr) Reference temp = 25 C 298.15 K Temp in and out = Beta-PL Acrylic acid Phospholic acid 983.5733 22644.939 29.507199 22644.939 206.25001 0 206.250010 954.0661 12986.522 91.244413 0 170 C 443.15 K Reaction Pathway: C3H4O2(1) ---> C3H4O2 g AH -44.5 kJ 954.0661Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


