Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Hi. I need an expert help to calculate Structured packing based on the question given : Calculation of Structured packing : -packing -gas flow -liquid
Hi. I need an expert help to calculate 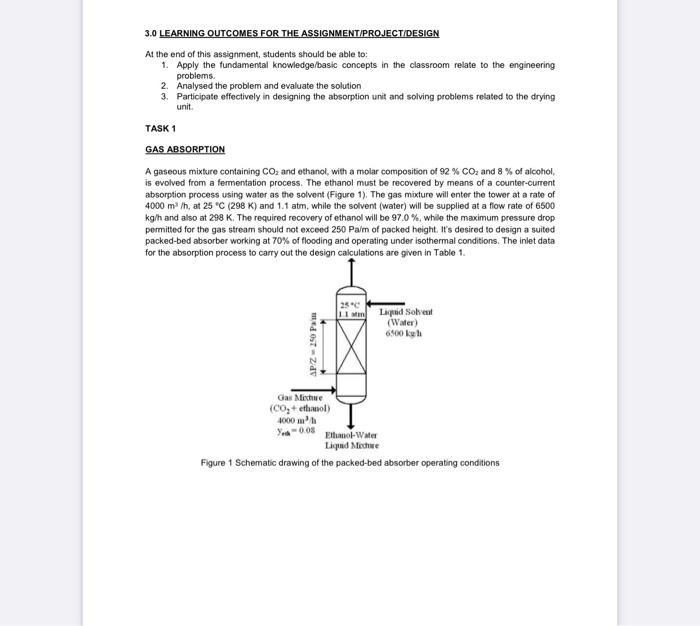
Structured packing based on the question given :
Calculation of Structured packing :
-packing
-gas flow
-liquid flow
-Tower diameter.
Please help me to do this. Please please please don't provide me any repeating answer i will dislike.
Please only answer if you can get the answer right. Thank you


I just need the calculation part that i have listed. Not based on the questions below
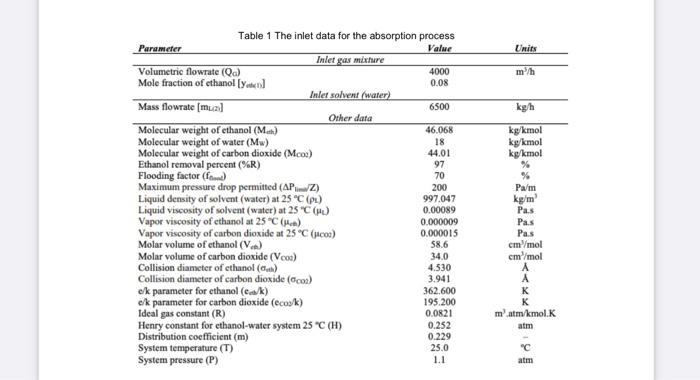
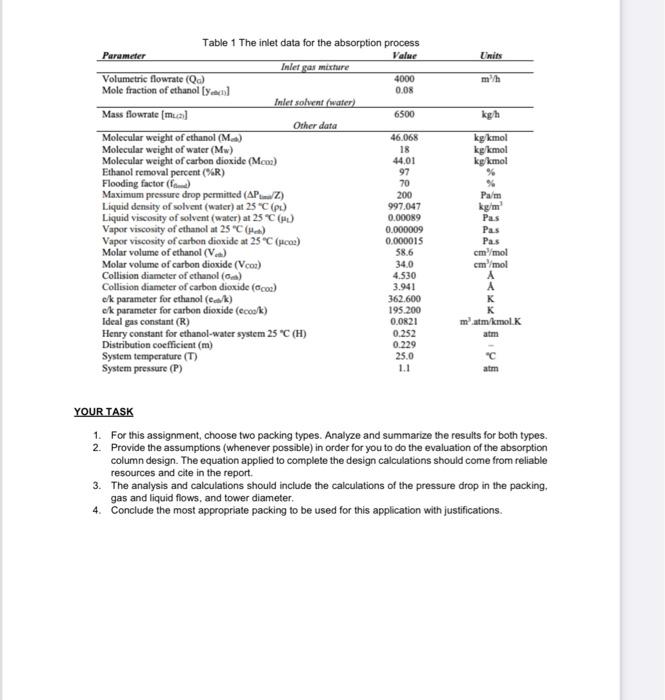
3.0 LEARNING OUTCOMES FOR THE ASSIGNMENT PROJECT/DESIGN At the end of this assignment, students should be able to: 1. Apply the fundamental knowledge/basic concepts in the classroom relate to the engineering problems. 2. Analysed the problem and evaluate the solution 3. Participate effectively in designing the absorption unit and solving problems related to the drying unit TASK 1 GAS ABSORPTION A gaseous mixture containing CO2 and ethanol, with a molar composition of 92% CO2 and 8 % of alcohol, is evolved from a fermentation process. The ethanol must be recovered by means of a counter-current absorption process using water as the solvent (Figure 1). The gas mixture will enter the tower at a rate of 4000 m/h, at 25 C (298 K) and 1.1 atm, while the solvent (water) will be supplied at a flow rate of 6500 kg/h and also at 298 K. The required recovery of ethanol will be 97.0 %, while the maximum pressure drop permitted for the gas stream should not exceed 250 Pa/m of packed height. It's desired to design a suited packed-bed absorber working at 70% of flooding and operating under isothermal conditions. The inlet data for the absorption process to carry out the design calculations are given in Table 1 25C 11 Lapid Solvent (Water) 6500 kul LOTZ Gas Mieste (C0+ ethol Ethanol-Water Liquid Meche Figure 1 Schematic drawing of the packed-bed absorber operating conditions Units m/h kg h kg/kmol kg/kmol kg/kmo! Table 1 The inlet data for the absorption process Parameter Value Inlet gas mixture Volumetric flowrate (Qc) 4000 Mole fraction of ethanol [ Yu] 0.08 Inlet solvent (water) Mass flowrate (mual 6500 Other data Molecular weight of ethanol (Main) 46.06% Molecular weight of water (Mw) 18 Molecular weight of carbon dioxide (Mcos) 44.01 Ethanol removal percent (%R) 97 Flooding factor (f) 70 Maximum pressure drop permitted (AP/Z) 200 Liquid density of solvent (water) at 25 C (PL) 997.047 Liquid viscosity of solvent (water) at 25 CM 0.00089 Vapor viscosity of ethanol at 25C) 0.000009 Vapor viscosity of carbon dioxide at 25C (ico) 0.000015 Molar volume of ethanol (V) 58.6 Molar volume of carbon dioxide (Vcoa) 34.0 Collision diameter of ethanol (0) 4.530 Collision diameter of carbon dioxide (2) 3.941 c/k parameter for ethanol (ek) 362.600 ok parameter for carbon dioxide (ecook) 195.200 Ideal gas constant (R) 0.0821 Henry constant for ethanol-water system 25 C (1) 0.252 Distribution coefficient (m) 0.229 System temperature (T) 25.0 System pressure (P) 1.1 Pa/m kg'm Pas Pas Pas cm /mol cm /mol matm/kmol. atm KAKKm-v atm Value Units mh kgh kg kmal kg/kmol kg kmal Table 1 The inlet data for the absorption process Parameter Inlet gas mixture Volumetric flowrate (Qc) 4000 Mole fraction of ethanol [y] 0.08 Inlet solvent (water) Mass flowrate (mual 6500 Other data Molecular weight of ethanol (M.) 46.068 Molecular weight of water (Mw) 18 Molecular weight of carbon dioxide (MCO) 44,01 Ethanol removal percent (R) 97 Flooding factor (f) 70 Maximum pressure drop permitted (APZ) 200 Liquid density of solvent (water) at 25C () 997.047 Liquid viscosity of solvent (water) at 25C) 0.00019 Vapor viscosity of ethanol at 25C (-) 0.000009 Vapor viscosity of carbon dioxide at 25C (cm) 0.000015 Molar volume of ethanol (V.) 58.6 Molar volume of carbon dioxide (VCO) 34.0 Collision diameter of ethanol (6) 4.530 Collision diameter of carbon dioxide (Co) 3.941 e/k parameter for ethanol (cak) 362.600 ck parameter for carbon dioxide (ccook) 195.200 Ideal gas constant (R) 0.0821 Henry constant for ethanol-water system 25 C (H) 0.252 Distribution coefficient (m) 0.229 System temperature (T) 25.0 System pressure (P) 1.1 Pa/m kg/ml Pas Pas Pas cm/mol cm /mol matm/kmol. atm "C atm YOUR TASK 1. For this assignment, choose two packing types. Analyze and summarize the results for both types. 2. Provide the assumptions (whenever possible) in order for you to do the evaluation of the absorption column design. The equation applied to complete the design calculations should come from reliable resources and cite in the report, 3. The analysis and calculations should include the calculations of the pressure drop in the packing, gas and liquid flows, and tower diameter. 4. Conclude the most appropriate packing to be used for this application with justifications. 3.0 LEARNING OUTCOMES FOR THE ASSIGNMENT PROJECT/DESIGN At the end of this assignment, students should be able to: 1. Apply the fundamental knowledge/basic concepts in the classroom relate to the engineering problems. 2. Analysed the problem and evaluate the solution 3. Participate effectively in designing the absorption unit and solving problems related to the drying unit TASK 1 GAS ABSORPTION A gaseous mixture containing CO2 and ethanol, with a molar composition of 92% CO2 and 8 % of alcohol, is evolved from a fermentation process. The ethanol must be recovered by means of a counter-current absorption process using water as the solvent (Figure 1). The gas mixture will enter the tower at a rate of 4000 m/h, at 25 C (298 K) and 1.1 atm, while the solvent (water) will be supplied at a flow rate of 6500 kg/h and also at 298 K. The required recovery of ethanol will be 97.0 %, while the maximum pressure drop permitted for the gas stream should not exceed 250 Pa/m of packed height. It's desired to design a suited packed-bed absorber working at 70% of flooding and operating under isothermal conditions. The inlet data for the absorption process to carry out the design calculations are given in Table 1 25C 11 Lapid Solvent (Water) 6500 kul LOTZ Gas Mieste (C0+ ethol Ethanol-Water Liquid Meche Figure 1 Schematic drawing of the packed-bed absorber operating conditions Units m/h kg h kg/kmol kg/kmol kg/kmo! Table 1 The inlet data for the absorption process Parameter Value Inlet gas mixture Volumetric flowrate (Qc) 4000 Mole fraction of ethanol [ Yu] 0.08 Inlet solvent (water) Mass flowrate (mual 6500 Other data Molecular weight of ethanol (Main) 46.06% Molecular weight of water (Mw) 18 Molecular weight of carbon dioxide (Mcos) 44.01 Ethanol removal percent (%R) 97 Flooding factor (f) 70 Maximum pressure drop permitted (AP/Z) 200 Liquid density of solvent (water) at 25 C (PL) 997.047 Liquid viscosity of solvent (water) at 25 CM 0.00089 Vapor viscosity of ethanol at 25C) 0.000009 Vapor viscosity of carbon dioxide at 25C (ico) 0.000015 Molar volume of ethanol (V) 58.6 Molar volume of carbon dioxide (Vcoa) 34.0 Collision diameter of ethanol (0) 4.530 Collision diameter of carbon dioxide (2) 3.941 c/k parameter for ethanol (ek) 362.600 ok parameter for carbon dioxide (ecook) 195.200 Ideal gas constant (R) 0.0821 Henry constant for ethanol-water system 25 C (1) 0.252 Distribution coefficient (m) 0.229 System temperature (T) 25.0 System pressure (P) 1.1 Pa/m kg'm Pas Pas Pas cm /mol cm /mol matm/kmol. atm KAKKm-v atm Value Units mh kgh kg kmal kg/kmol kg kmal Table 1 The inlet data for the absorption process Parameter Inlet gas mixture Volumetric flowrate (Qc) 4000 Mole fraction of ethanol [y] 0.08 Inlet solvent (water) Mass flowrate (mual 6500 Other data Molecular weight of ethanol (M.) 46.068 Molecular weight of water (Mw) 18 Molecular weight of carbon dioxide (MCO) 44,01 Ethanol removal percent (R) 97 Flooding factor (f) 70 Maximum pressure drop permitted (APZ) 200 Liquid density of solvent (water) at 25C () 997.047 Liquid viscosity of solvent (water) at 25C) 0.00019 Vapor viscosity of ethanol at 25C (-) 0.000009 Vapor viscosity of carbon dioxide at 25C (cm) 0.000015 Molar volume of ethanol (V.) 58.6 Molar volume of carbon dioxide (VCO) 34.0 Collision diameter of ethanol (6) 4.530 Collision diameter of carbon dioxide (Co) 3.941 e/k parameter for ethanol (cak) 362.600 ck parameter for carbon dioxide (ccook) 195.200 Ideal gas constant (R) 0.0821 Henry constant for ethanol-water system 25 C (H) 0.252 Distribution coefficient (m) 0.229 System temperature (T) 25.0 System pressure (P) 1.1 Pa/m kg/ml Pas Pas Pas cm/mol cm /mol matm/kmol. atm "C atm YOUR TASK 1. For this assignment, choose two packing types. Analyze and summarize the results for both types. 2. Provide the assumptions (whenever possible) in order for you to do the evaluation of the absorption column design. The equation applied to complete the design calculations should come from reliable resources and cite in the report, 3. The analysis and calculations should include the calculations of the pressure drop in the packing, gas and liquid flows, and tower diameter. 4. Conclude the most appropriate packing to be used for this application with justifications Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


