Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Hi , ive been trying so find a way to calculate this but im very unsure how. And i see that M(mg) is missing so
Hi , ive been trying so find a way to calculate this but im very unsure how. And i see that M(mg) is missing so you could just set a number there. I just want to know the formulas of this type of problem
Thanks!
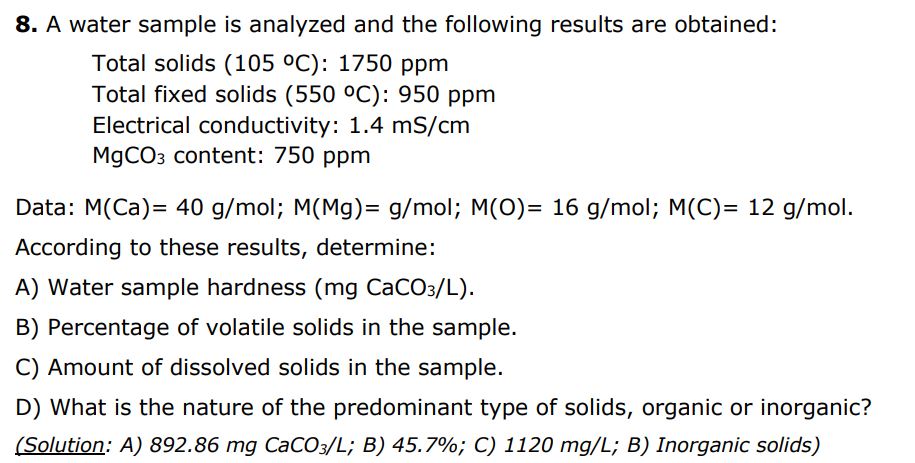
8. A water sample is analyzed and the following results are obtained: Total solids (105C):1750ppm Total fixed solids (550C):950ppm Electrical conductivity: 1.4mS/cm MgCO3 content: 750ppm Data: M(Ca)=40g/mol;M(Mg)=g/mol;M(O)=16g/mol;M(C)=12g/mol. According to these results, determine: A) Water sample hardness ( mgCaCO3/L). B) Percentage of volatile solids in the sample. C) Amount of dissolved solids in the sample. D) What is the nature of the predominant type of solids, organic or inorganic? (Solution: A) 892.86mgCaCO3/L; B) 45.7%; C) 1120mg/L; B) Inorganic solids)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


