Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Hi, please answer all parts it will be much appreacited. (a) You are investigating a decomposition reaction over a heterogeneous catalyst. Explain how you could
Hi, please answer all parts it will be much appreacited. 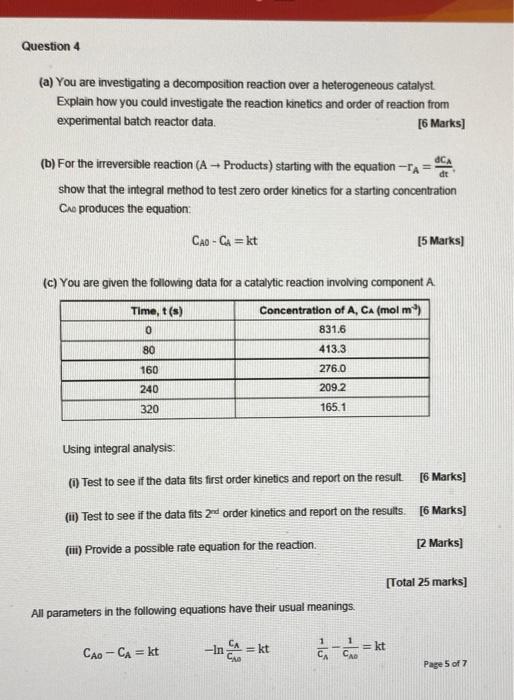
(a) You are investigating a decomposition reaction over a heterogeneous catalyst. Explain how you could investigate the reaction kinetics and order of reaction from experimental batch reactor data. [6 Marks] (b) For the irreversible reaction (A Products) starting with the equation rA=dtdCA, show that the integral method to test zero order kinetics for a starting concentration Cuo produces the equation: CAOCAA=kt [5 Marks] (c) You are given the following data for a catalytic reaction involving component A. Using integral analysis: (i) Test to see if the data fits first order kinetics and report on the result. [6 Marks] (ii) Test to see if the data fits 2ad order kinetics and report on the results. [6 Marks] (iii) Provide a possible rate equation for the reaction. [2 Marks] [Total 25 marks] All parameters in the following equations have their usual meanings. CAOCA=ktlnCAOCA=ktCA1CA01=kt Page 5 of 7 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


