Answered step by step
Verified Expert Solution
Question
1 Approved Answer
What is the solubility product constant for Ba(103)2 that has a molar solubility of 3.93 x 10 5 M in 0.02 M of Ba(NO,),




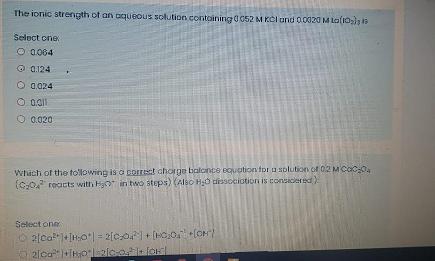
What is the solubility product constant for Ba(103)2 that has a molar solubility of 3.93 x 10 5 M in 0.02 M of Ba(NO,), solution? Select one O 1.6 x 109 O 12 x 1010* O 2.2 x 10 O 1.0x 1011 2.0 x 10-5 The activity of Zn2+ in a soturated solution with Zn(OH), with 0.00500 M KNO, (Ksp 1.20 x107 Y zn = 0.748, Y OH = 0.926) is: Select one O 265x10 O 865x10 4.50X10 O 545x10 O 125 X106 The ion product constant of pure water ot 30 C with pH value of 6.92 is Select one. O 5.50 x 1014 2.88 x 1015 O 1.45 x1014 6.92 x 10-15 2.88 x 1014 The activity coefficient of Zn* with ionic strength of 0.083 mol/L using Debye-Huckel law (a Zn2* =0.6 nm) is: D-H equation is -Log yx= (051 72 Vu)/0+33oxvu) Select one: O 0.589 O 0.875 0.375 O 0.422 O 0.288 The ionic strength of an oqueous solution contoining0 052 M KCI und 0020 M La(10) Select cne O a064 O 0.124 O 0.024 O 0.020 Which of the tollowing is a porrest chorge balonte eguation for u solution of 02 MCac0, (C:0 reacts with Hyo in two steps) (Also H;0 tsscciotioni is consicered Select one
Step by Step Solution
★★★★★
3.63 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


