Question
Hints: 1) This problem specifies that the methanol concentration is 95 mole % in the distillate, and 5 mole % in the bottoms. This makes
Hints: 1) This problem specifies that the methanol concentration is 95 mole % in the distillate, and 5 mole % in the bottoms. This makes methanol the light key. 2) The heavy key is not specified, but this would be the next heaviest component, ethanol. For the initial distillate and bottoms composition estimate, assume that the 5 mole % of the distillate that is not methanol is ethanol. 3) In the equations and examples given in class, the reference compound for bubble point, dew point, and relative volatility calculations was always compound C. For this problem, the heavy key is ethanol, compound B, so B should be the reference for bubble point, dew point, and relative volatility calculations. (For an ideal system Ki=Pi/P).
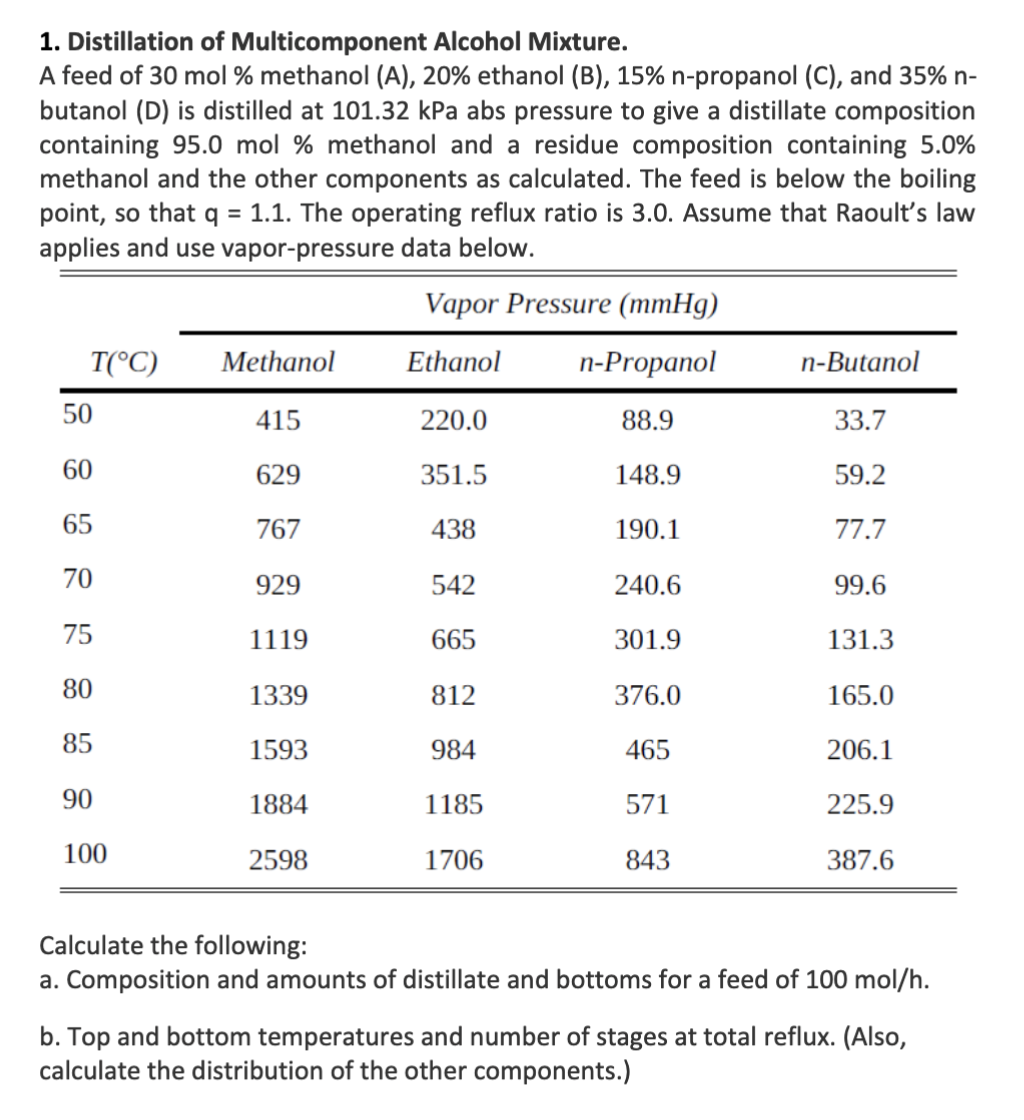
1. Distillation of Multicomponent Alcohol Mixture. A feed of 30 mol % methanol (A), 20% ethanol (B), 15% n-propanol (C), and 35% n- butanol (D) is distilled at 101.32 kPa abs pressure to give a distillate composition containing 95.0 mol % methanol and a residue composition containing 5.0% methanol and the other components as calculated. The feed is below the boiling point, so that q = 1.1. The operating reflux ratio is 3.0. Assume that Raoult's law applies and use vapor-pressure data below. Vapor Pressure (mmHg) T(C) Methanol Ethanol n-Propanol n-Butanol 50 415 220.0 88.9 33.7 60 629 351.5 148.9 59.2 65 767 438 190.1 77.7 70 929 542 240.6 99.6 75 1119 665 301.9 131.3 80 1339 812 376.0 165.0 85 1593 984 465 206.1 90 1884 1185 571 225.9 100 2598 1706 843 387.6 Calculate the following: a. Composition and amounts of distillate and bottoms for a feed of 100 mol/h. b. Top and bottom temperatures and number of stages at total reflux. (Also, calculate the distribution of the other components.)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


