Answered step by step
Verified Expert Solution
Question
1 Approved Answer
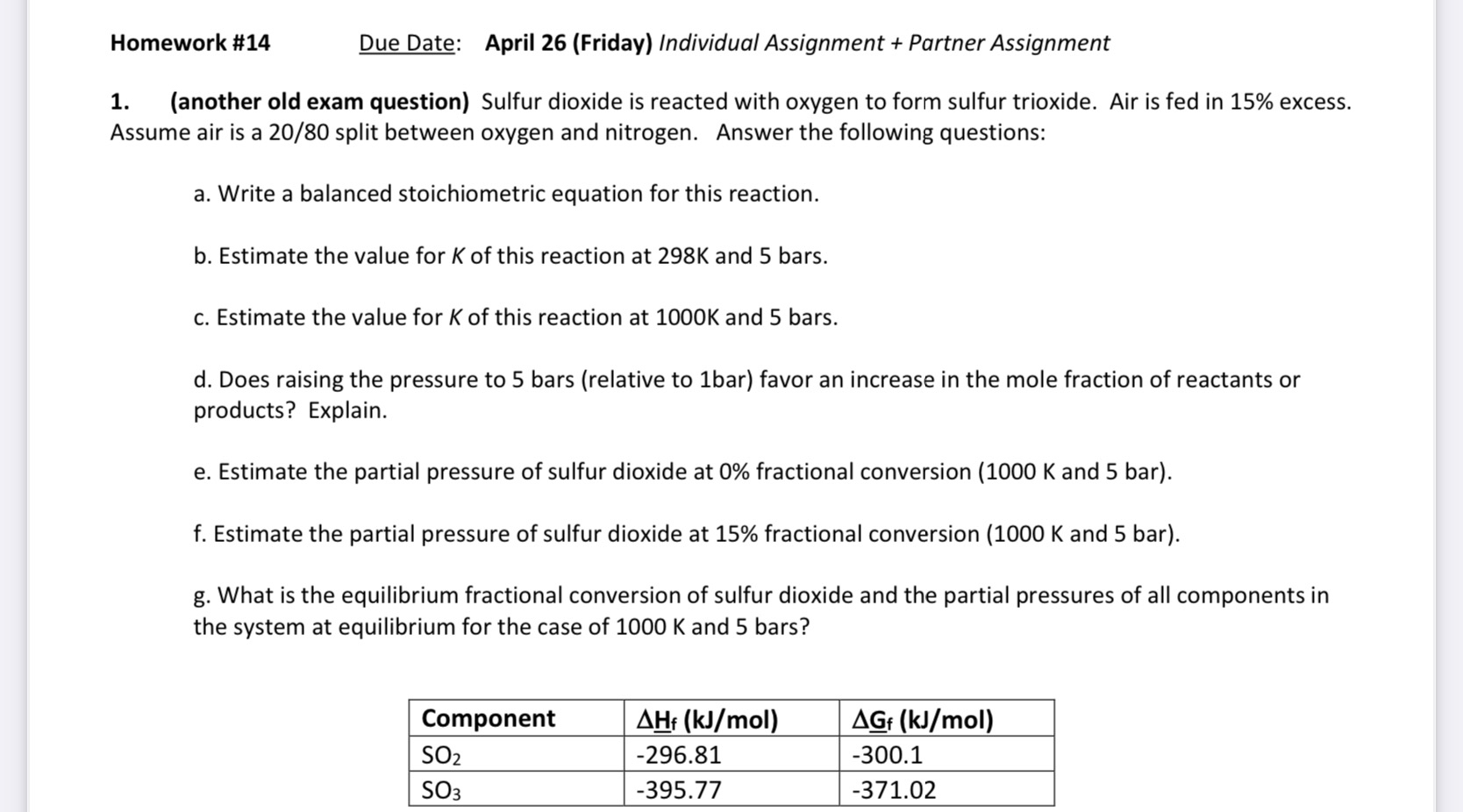
Homework # 1 4 Due Date: April 2 6 ( Friday ) Individual Assignment + Partner Assignment ( another old exam question ) Sulfur dioxide
Homework #
Due Date: April Friday Individual Assignment Partner Assignment
another old exam question Sulfur dioxide is reacted with oxygen to form sulfur trioxide. Air is fed in excess. Assume air is a split between oxygen and nitrogen. Answer the following questions:
a Write a balanced stoichiometric equation for this reaction.
b Estimate the value for of this reaction at and bars.
c Estimate the value for of this reaction at and bars.
d Does raising the pressure to bars relative to bar favor an increase in the mole fraction of reactants or products? Explain.
e Estimate the partial pressure of sulfur dioxide at fractional conversion and bar
f Estimate the partial pressure of sulfur dioxide at fractional conversion and bar
g What is the equilibrium fractional conversion of sulfur dioxide and the partial pressures of all components in the system at equilibrium for the case of and bars?
tableComponent
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


