Answered step by step
Verified Expert Solution
Question
1 Approved Answer
hopefully it's clear now Thermodytamick Analyais Ges Catalytic Dry Reflomeng of Mlethane as Synthests Gas This progect mat include the followity enctions: Summary Table ef

hopefully it's clear now 
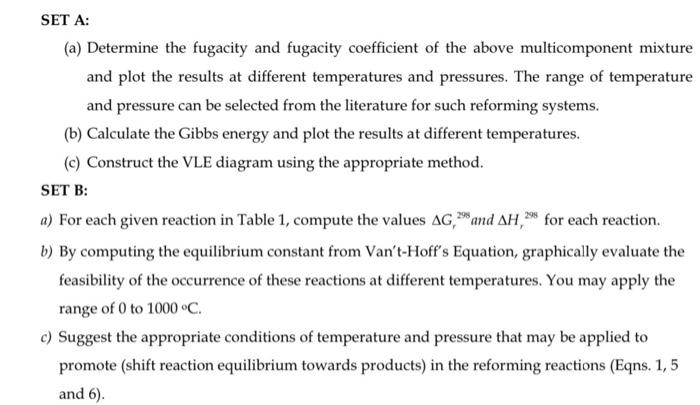
Thermodytamick Analyais Ges Catalytic Dry Reflomeng of Mlethane as Synthests Gas This progect mat include the followity enctions: Summary Table ef ceatents 1. Introductice 2. Methodeloyry 3. Thentodyamics model 4. Revales and discussion 5. Chnclusion 6. Aeferences Description of Frejecti: CO4 and CH4 are the two main gres causing globul warning throwigh the groenhouse gas effect. They are also the main componertb of tioges (rowghly 35.50: CO2 and 5065=CH4). natural gas (roupdly 70-95" CH.), and tlue cas (maiety CON). Up-to-date, biogas is mootly used for heat or chectricity productiont. However, bocas can abe be trandormed into liquild fucls and chemicals by ditlerent exoleming procesoes, wach as saram noforming, dry exotming, and trireforming. The main feactions ane summariani belom: Table 1: SET A: (a) Determine the fugacity and fupicity coellicient of the above maltioomponent mixtufe and plot the results at dillerene tempertures and pressures. The range of temperature and pressure can be silectid from the lacrature for wuch refoening systems. (b) Cakulase the Gibbs energy and plot the resalts at different semperatures. (c) Construct the VLE diagram using the appoypriate method. SET B: a) For each piven reaction in Table 1, compote the valoe 3G, =mal AH, for each reation. b) Ey computing the equilibrium constant fnom Van'thoffs Equation graphically evaluaste the feasibility of the occurrence of these reations at difienent iemperature. You may apply the range of 0 to 1000C of Sussost the appopriate conditices of tempreitane and gresiane that may be applied to promote (shitt reaction equilifrium towards producty in the metorining mactions (Eqns. 1, 5 Thermodynamics Analysis for Catalytic Dry Reforming of Methane to Synthesis Gas This project must include the following sections: Summary Table of contents 1. Introduction 2. Methodology 3. Thermodynamics model 4. Results and discussion 5. Conclusions 6. References Description of Project: CO2 and CH4 are the two main gases causing global warming through the greenhouse gas effect. They are also the main components of biogas (roughly 3550%CO2 and 5065%CH4 ). natural gas (roughly 70-95\% CH4 ), and flue gas (mainly CO2 ). Up-to-date, biogas is mostly used for heat or electricity production. However, biogas can also be transformed into liquid fuels and chemicals by different reforming processes, such as steam reforming dry reforming and trireforming. The main reactions are summarized below: Table 1: SET A: (a) Determine the fugacity and fugacity coefficient of the above multicomponent mixture and plot the results at different temperatures and pressures. The range of temperature and pressure can be selected from the literature for such reforming systems. (b) Calculate the Gibbs energy and plot the results at different temperatures. (c) Construct the VLE diagram using the appropriate method. SET B: a) For each given reaction in Table 1, compute the values Gr298 and Hr298 for each reaction. b) By computing the equilibrium constant from Van't-Hoff's Equation, graphically evaluate the feasibility of the occurrence of these reactions at different temperatures. You may apply the range of 0 to 1000C. c) Suggest the appropriate conditions of temperature and pressure that may be applied to promote (shift reaction equilibrium towards products) in the reforming reactions (Eqns. 1, 5 and 6) 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


