Question
A 2.50 litres of boiling water (100 C) is poured into a 0.650 kg glass bowl that was initially at = 837 J.kg-.K- 24.0
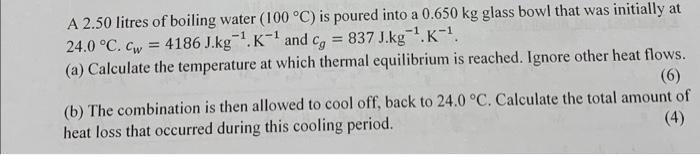
A 2.50 litres of boiling water (100 C) is poured into a 0.650 kg glass bowl that was initially at = 837 J.kg-.K- 24.0 C. Cw=4186 J.kg-. K- and cg -1 (a) Calculate the temperature at which thermal equilibrium is reached. Ignore other heat flows. (6) (b) The combination is then allowed to cool off, back to 24.0 C. Calculate the total amount of (4) heat loss that occurred during this cooling period.
Step by Step Solution
3.57 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1
Volume of water 25 Litres Temperature of water 100C Mass of water 25 kg When the thermal equilibrium ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Cornerstones of Managerial Accounting
Authors: Mowen, Hansen, Heitger
3rd Edition
324660138, 978-0324660135
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



