Answered step by step
Verified Expert Solution
Question
1 Approved Answer
How did we get H' 2 ? I don't know how they interpolated, please help me with this. Example 7.8 Saturated-vapor steam at 100kPa(tsat=99.63C) is

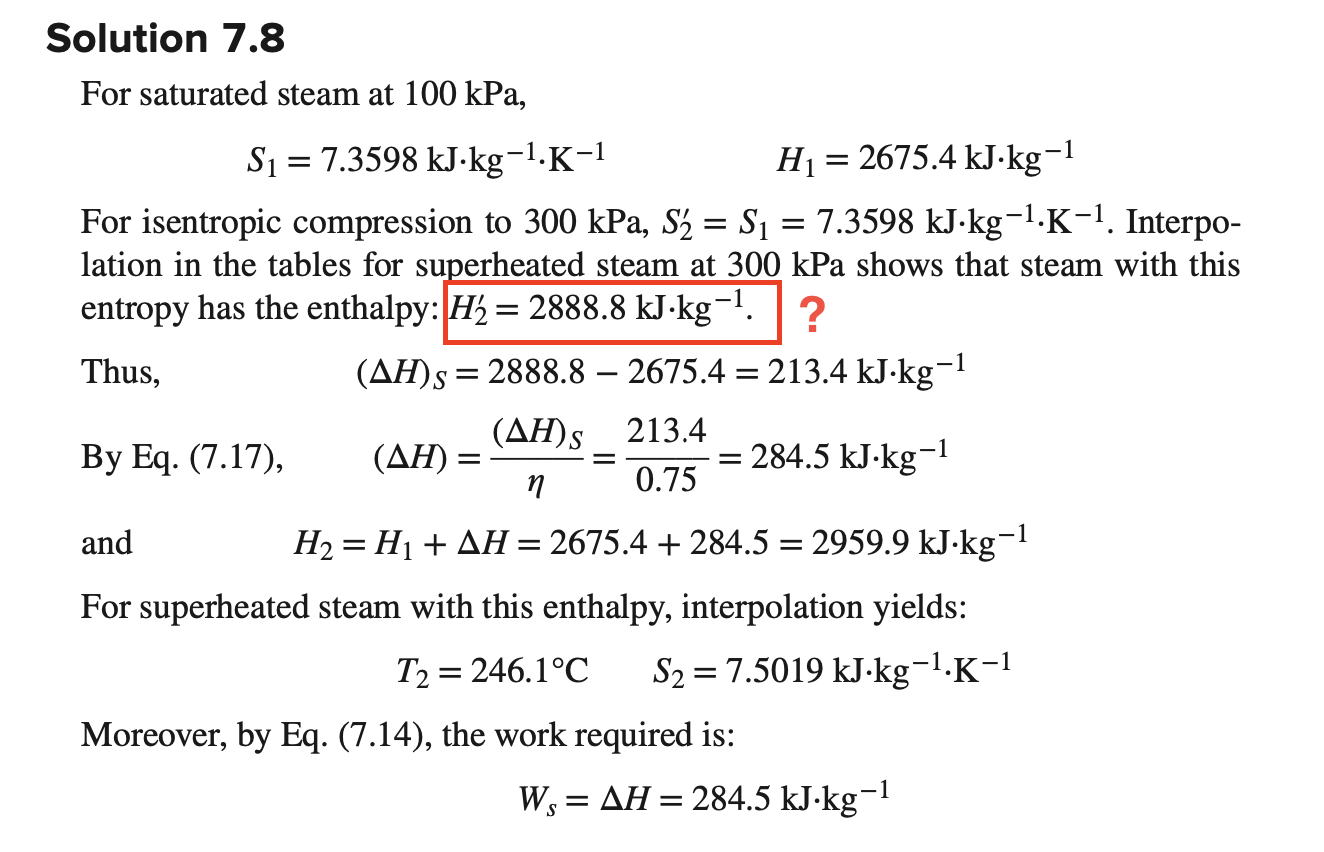
How did we get H'2? I don't know how they interpolated, please help me with this.
Example 7.8 Saturated-vapor steam at 100kPa(tsat=99.63C) is compressed adiabatically to 300kPa. If the compressor efficiency is 0.75, what is the work required and what are the properties of the discharge stream? For saturated steam at 100kPa, S1=7.3598kJkg1K1H1=2675.4kJkg1 For isentropic compression to 300kPa,S2=S1=7.3598kJkg1K1. Interpolation in the tables for superheated steam at 300kPa shows that steam with this entropy has the enthalpy: H2=2888.8kJkg1. ? By Eq. (7.17), (H)=(H)S=0.75213.4=284.5kJkg1 and H2=H1+H=2675.4+284.5=2959.9kJkg1 For superheated steam with this enthalpy, interpolation yields: T2=246.1CS2=7.5019kJkg1K1 Moreover, by Eq. (7.14), the work required is: Ws=H=284.5kJkg1Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


