Answered step by step
Verified Expert Solution
Question
1 Approved Answer
how do I find delta H for these? 6.6539g of NaNO3 was dissolved in 30.00g of water in a calorimeter at 23.0C, the temperature of
how do I find delta H for these? 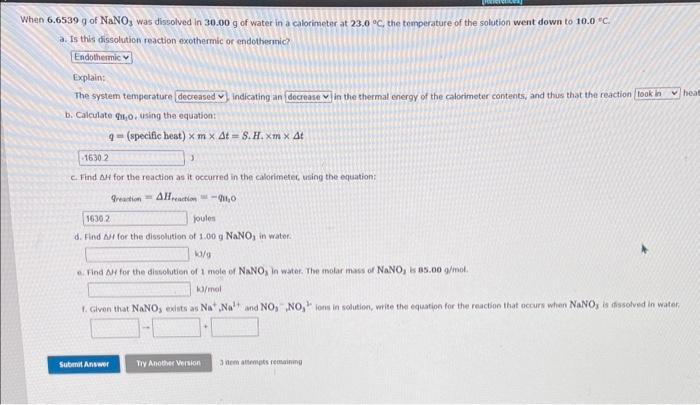
6.6539g of NaNO3 was dissolved in 30.00g of water in a calorimeter at 23.0C, the temperature of the solution went down to 10.0. C. a. Is this dissolution reaction exothermic or endothernic? Explain: The system temperature indicating an in the thermal energy of the calorimeter contents, and thus that the reaction b. Calculate Th 1. using the equation: q=(specificheat)mt=S+H+mt c. Find f for the reaction as it occurred in the calorimetec, using the equation: qreation=Hreaction=i3O jpules d. Find AM for the dissolution of 1.00gNaNO3 in water. ka/9 e. Find AH for the dissohtion of I nole of NaNO3 in water. The molar mass of NaNO3 is 85.009/m0m. k) fimal 1. Given that NaNO3 exists as Na+,Na3, and NO3NO33 ions in solution, write the equation for the reacbion that occurs when NaNO3 is ifsssolved in water. 3 arm attrmpts resaining 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


