Question
How do I find the K c & Delta G? The experiment is about the Empirical Formula and Free Energy of Formation of Iron(III) Salicylate
How do I find the Kc & Delta G?
The experiment is about the Empirical Formula and Free Energy of Formation of Iron(III) Salicylate Complex
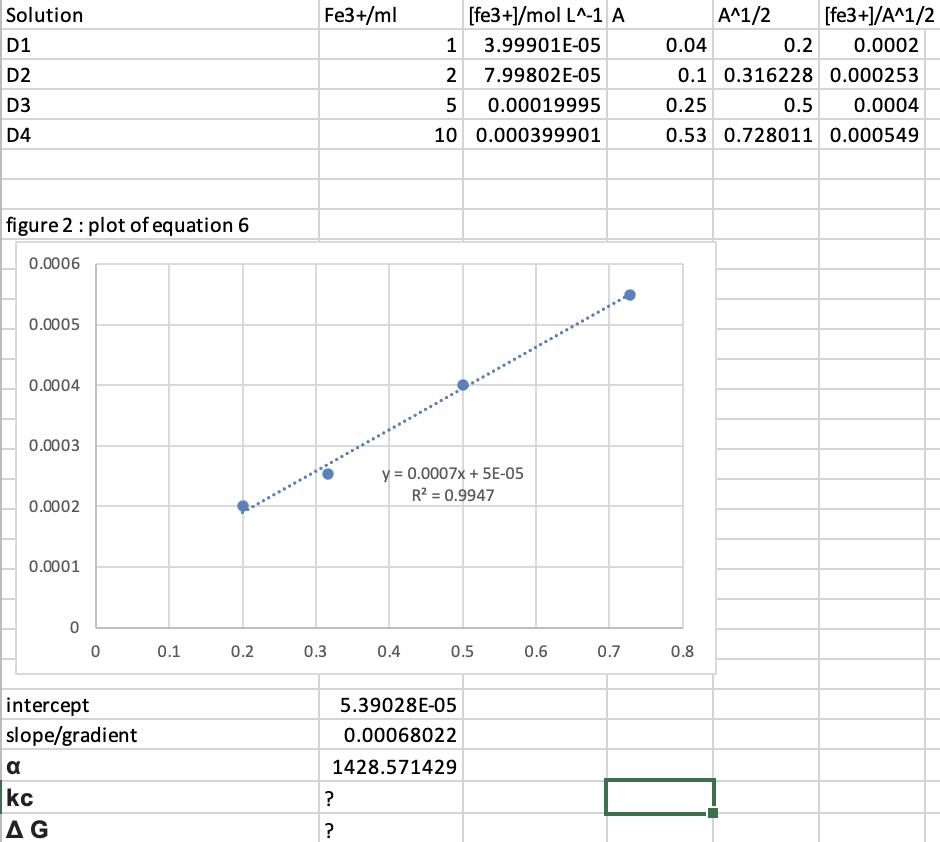
Solution D1 D2 D3 D4 figure 2: plot of equation 6 0.0006 0.0005 0.0004 0.0003 0.0002 0.0001 0 a kc AG 0 intercept slope/gradient 0.1 0.2 Fe3+/ml 0.3 ? ? 0.4 [fe3+]/mol L^-1 A 1 3.99901E-05 2 7.99802E-05 5 0.00019995 10 0.000399901 y = 0.0007x + 5E-05 R = 0.9947 5.39028E-05 0.00068022 1428.571429 0.5 0.6 0.7 A^1/2 0.8 [fe3+]/A^1/2 0.0002 0.04 0.2 0.1 0.316228 0.000253 0.25 0.5 0.0004 0.53 0.728011 0.000549
Step by Step Solution
3.37 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1
The Kc and Delta G of a reaction can be found using thermodynamic data Thermodynamic data can be obtained from chemical databases such as the NIST Che...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry The Central Science
Authors: Theodore Brown, Eugene LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward
12th edition
321696727, 978-0132175081, 978-0321696724
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



