Answered step by step
Verified Expert Solution
Question
1 Approved Answer
How do we go about this? A chemical plant generates 2 2 0 0 k g mol / ? h of flue gas containing 7
How do we go about this?
A chemical plant generates mol of flue gas containing mol pollutant that
must be reduced to less than mol before the gas can be released to the
atmosphere. You are asked to design a gas scrubber absorber to clean up the pollutant in the flue
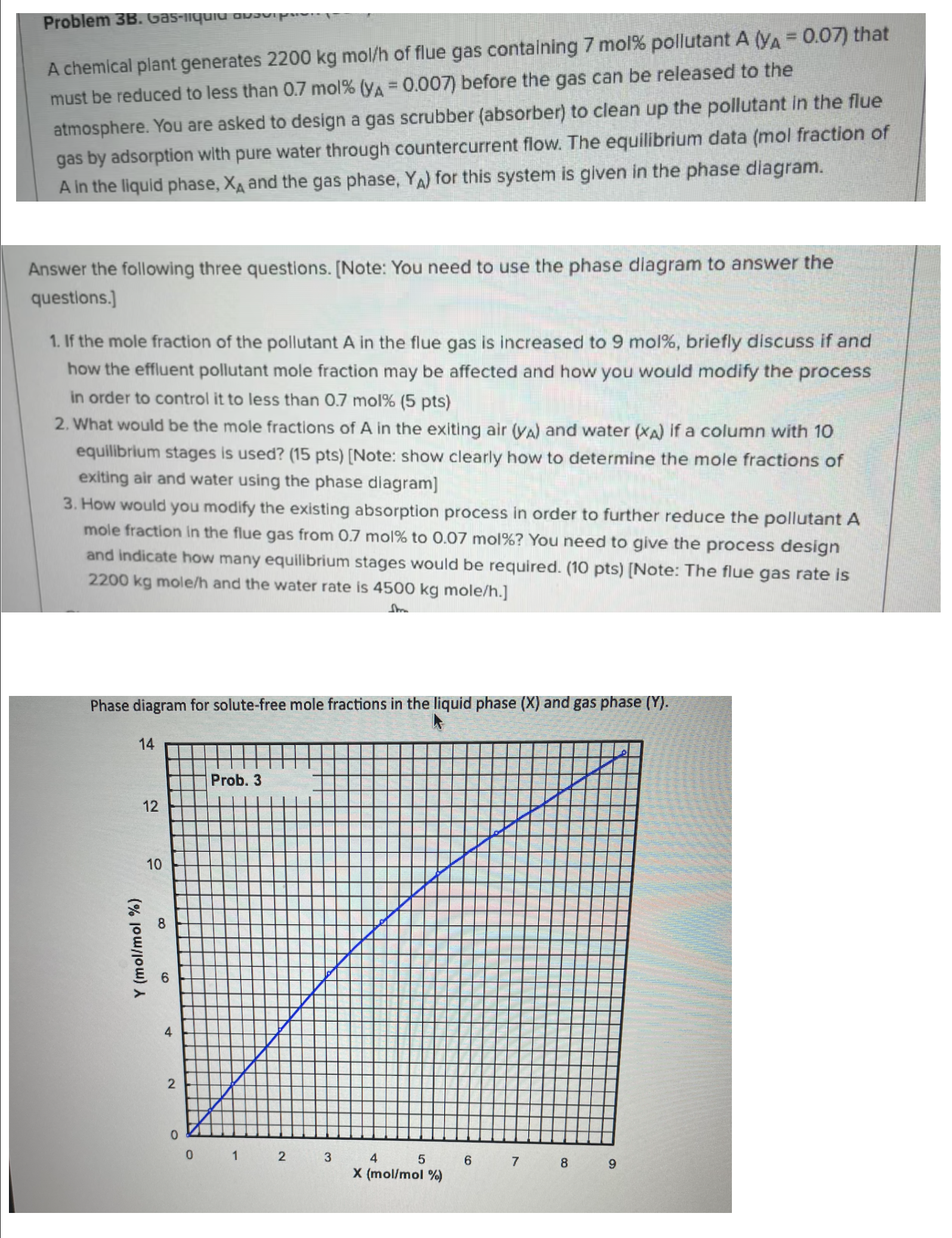
gas by adsorption with pure water through countercurrent flow. The equilibrium data mol fraction of
in the liquid phase, and the gas phase, for this system is given in the phase diagram.
Answer the following three questions. Note: You need to use the phase dlagram to answer the
questions.
If the mole fraction of the pollutant in the flue gas is increased to mol briefly discuss if and
how the effluent pollutant mole fraction may be affected and how you would modify the process
in order to control it to less than mol pts
What would be the mole fractions of in the exiting air and water if a column with
equilibrium stages is used? Note: show clearly how to determine the mole fractions of
exiting air and water using the phase dlagram
How would you modify the existing absorption process in order to further reduce the pollutant
mole fraction in the flue gas from mol to mol You need to give the process design
and indicate how many equilibrium stages would be required. ptsNote: The flue gas rate is
kgmol and the water rate is moleh
Phase diagram for solutefree mole fractions in the liquid phase and gas phase
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


