Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Convert the mass of fat in your first three trials to moles using the following equation. Record your values in Data Table 1. moles


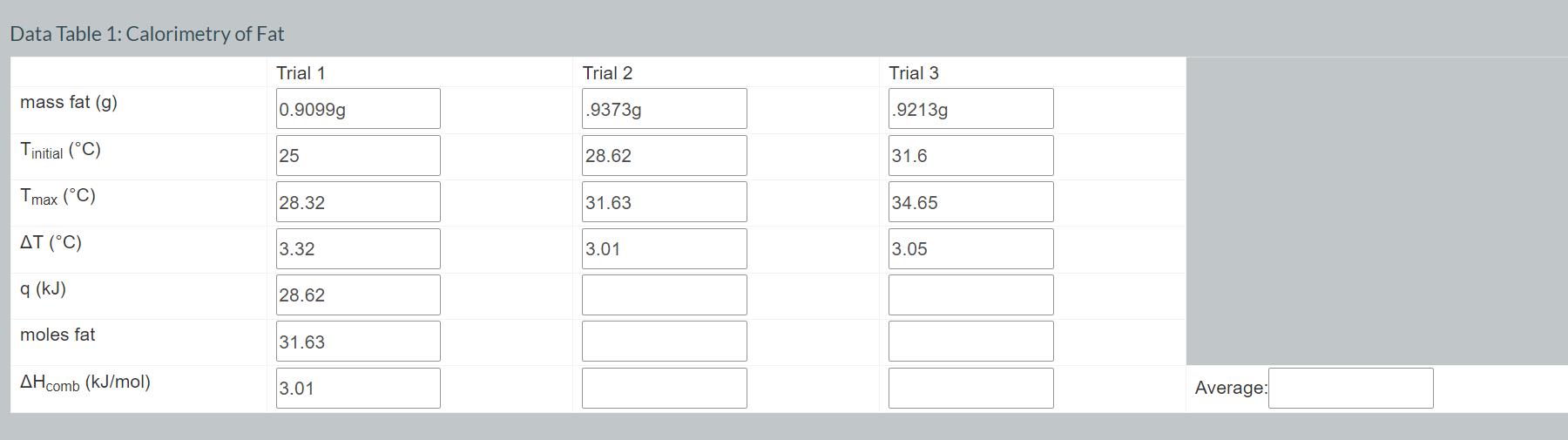
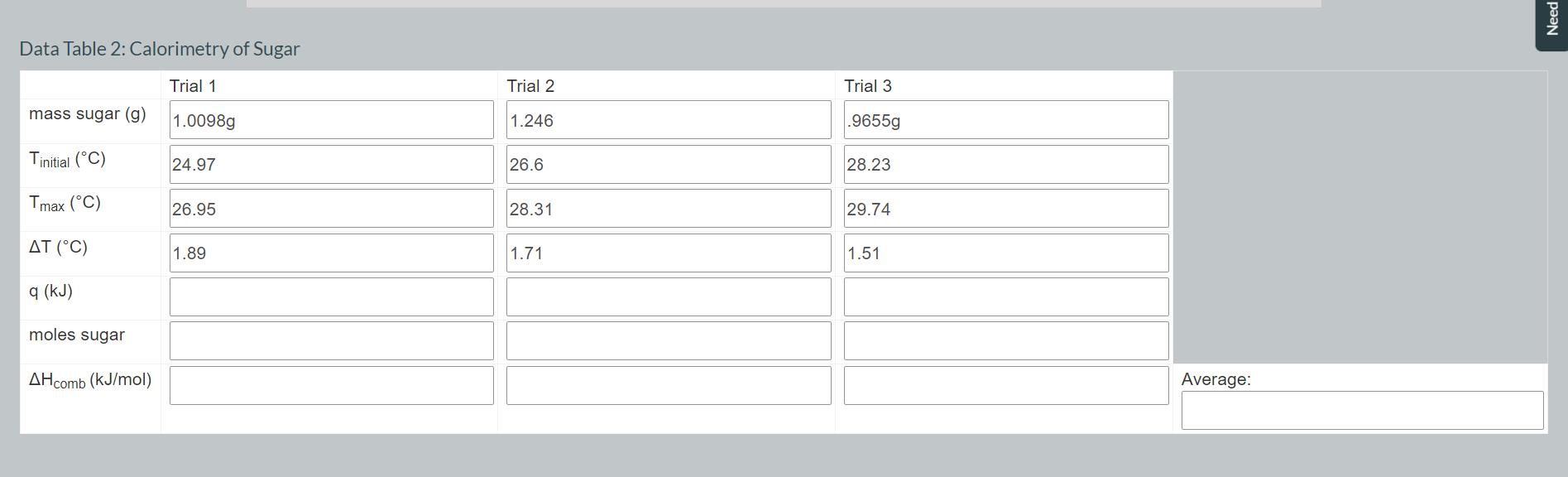
Convert the mass of fat in your first three trials to moles using the following equation. Record your values in Data Table 1. moles fat g fat. = 1 mol fat 797.7 g fat Convert the mass of sugar in your second three trials to moles using the following equation. Record your values in Data Table 2. 1 mol sugar moles sugar = x g sugar. 342.3 g sugar Using your values for the number of moles of fat or sugar and q. calculate the heat of combustion in kJ/mol for each of your trials using the following equation. Record your values in Data Tables 1 and 2. Average your values of AHcomb for fat and record in Data Table 1. Average your values of AH comb for sugar and record in Data Table 2. Data Table 1: Calorimetry of Fat mass fat (g) Tinitial (C) Tmax (C) AT (C) q (kJ) Trial 1 Trial 2 0.9099g .9373g Trial 3 .9213g 25 28.62 31.6 28.32 31.63 34.65 3.32 3.01 3.05 28.62 moles fat 31.63 AHcomb (kJ/mol) 3.01 Average: Data Table 2: Calorimetry of Sugar Trial 1 Trial 2 Trial 3 mass sugar (g) 1.0098g Tinitial (C) 24.97 1.246 26.6 .9655g 28.23 Tmax (C) 26.95 28.31 29.74 (C) 1.89 1.71 1.51 q (kJ) moles sugar AHcomb (kJ/mol) Average: Need
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


