Answered step by step
Verified Expert Solution
Question
1 Approved Answer
how do you calculate Hess's law? Hess's Law Calculation: Refer to reactions in introduction. H1 Mg(s)+HCl(aq)MHOl2(aq)+H2g H MgO(s)+HCl(aq) H3=285.9kJ/mol H2(g)+1/2O2(g)H2O(()(heatofformationofwater) Combine H1,H2&H3 using Hess's law

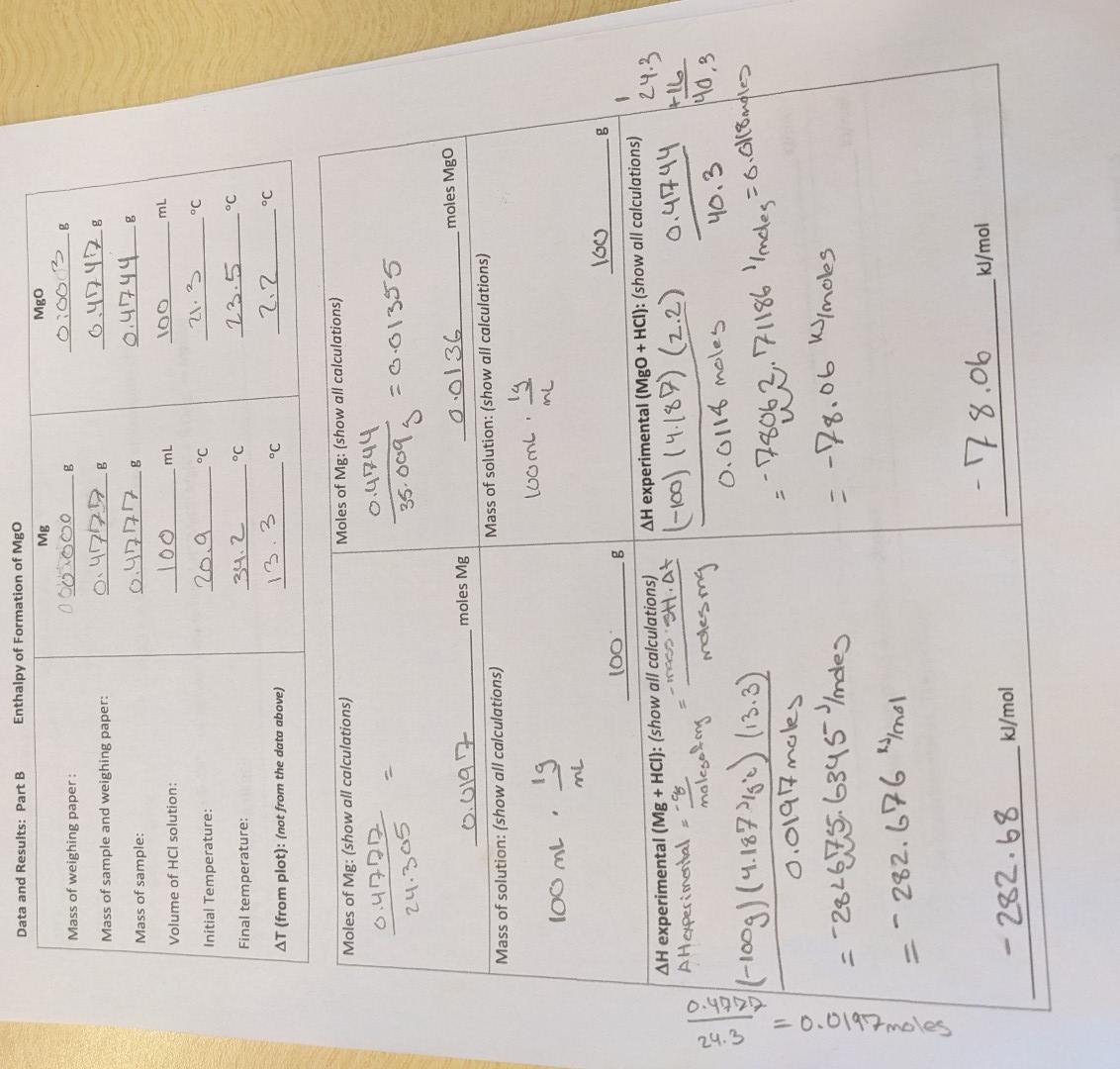

how do you calculate Hess's law?
Hess's Law Calculation: Refer to reactions in introduction. H1 Mg(s)+HCl(aq)MHOl2(aq)+H2g H MgO(s)+HCl(aq) H3=285.9kJ/mol H2(g)+1/2O2(g)H2O(()(heatofformationofwater) Combine H1,H2&H3 using Hess's law to find HH(MgO):Mg(s)+1/2O2(g)MgO(s) Data and Results: Part B Enthalpy of Formation of MgO In part B of this experiment, we will be finding the enthalpy of formation for MgO(s) using an indirect method. According to Hess's Law, if two or more reactions can be added to give a net reaction, H for the net reaction is simply the sum of the Hs for the reactions which are added. Consider the following three reactions: (1) Mg(s)+2H+(aq)Mg2+(aq)+H2(g)H1 (2) Mg+2(aq)+H2O(l)MgO(s)+2H+(aq)H2 (3) H2(g)+1/2O2(g)H2O(l) HH3 (4) Mg(s)+1/2O2 (g) MgO(s)H4=H1+H2+H3 Notice that when the three reactions are added, they form the formation reaction for MgO. Analogously, when we add the enthalpies for the three reactions, they give the enthalpy change associated with the formation reactionStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


