Question
What is the specific heat capacity of the metal? How many grams of water would require 92.048 kJ of heat to raise its temperature from
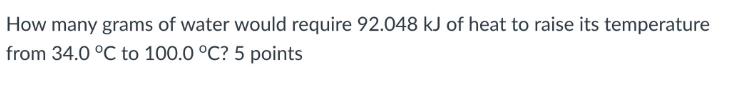

What is the specific heat capacity of the metal?
How many grams of water would require 92.048 kJ of heat to raise its temperature from 34.0 C to 100.0 C? 5 points
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Answer To calculate the heat required to raise the temperature of a substance ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Financial Reporting Financial Statement Analysis And Valuation A Strategic Perspective
Authors: James M. Wahlen, Stephen P. Baginski, Mark Bradshaw
9th Edition
1337614689, 1337614688, 9781337668262, 978-1337614689
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



