Answered step by step
Verified Expert Solution
Question
1 Approved Answer
how to calulate capillart force Simulation: Compound Radius of capillary tube (mm) Height of liquid in tubing (mm) Calculated Capillary force (Newtons) Acetone (2-propanone) Benzene
how to calulate capillart force 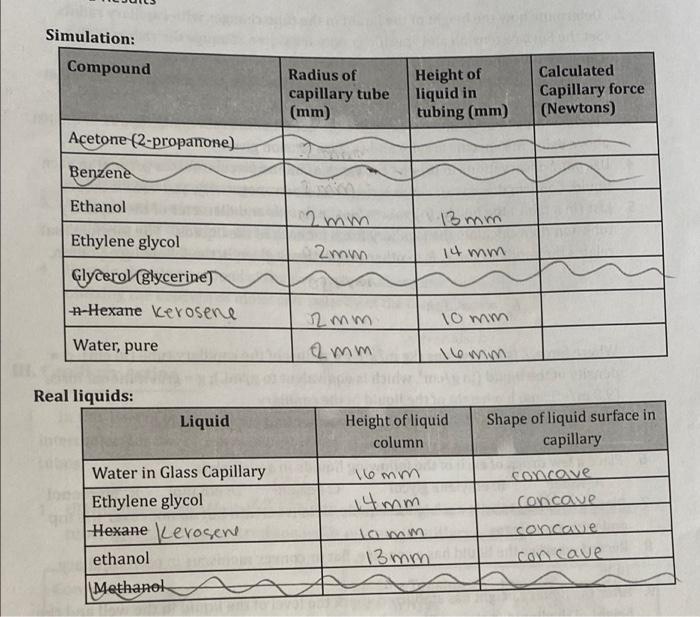
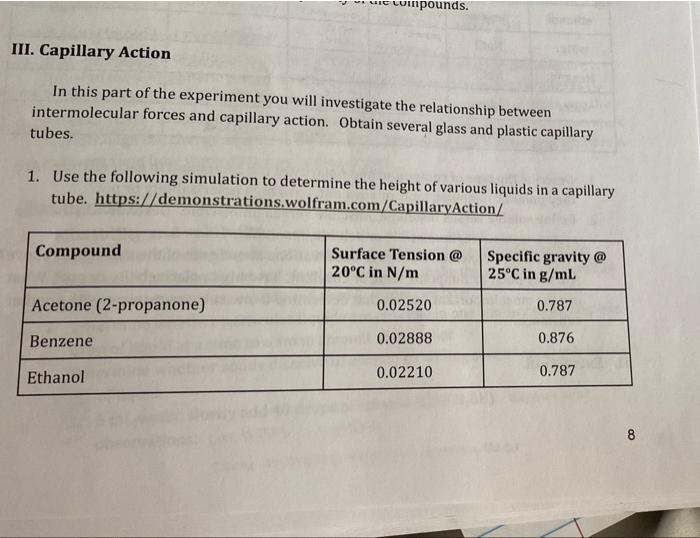

Simulation: Compound Radius of capillary tube (mm) Height of liquid in tubing (mm) Calculated Capillary force (Newtons) Acetone (2-propanone) Benzene Ethanol 2 mm 13 mm Ethylene glycol 2mm 14 mm Glycerol (glycerine) n-Hexane Kerosene 10 mm 12mm 2 mm Water, pure lemm Real liquids: Liquid Water in Glass Capillary Ethylene glycol Hexane Lerosene ethanol Methanol Height of liquid Shape of liquid surface in column capillary 16mm concave 14mm concave lamm concowe 13mm concave a coinpounds. III. Capillary Action In this part of the experiment you will investigate the relationship between intermolecular forces and capillary action. Obtain several glass and plastic capillary tubes. 1. Use the following simulation to determine the height of various liquids in a capillary tube. https://demonstrations.wolfram.com/CapillaryAction/ Compound Surface Tension @ 20C in N/m Specific gravity @ 25C in g/ml Acetone (2-propanone) 0.02520 0.787 Benzene 0.02888 0.876 Ethanol 0.02210 0.787 8 Ethylene glycol Glycerol (glycerine) n-Hexane 0.04770 1.100 0.06400 1.263 Water, pure 0.01843 0.657 0.07280 1.000 2. Determine how the height of the liquid in the tube changes with capillary radius. Set the Surface tension and specific gravity for water. Change the radius of the capillary tube and record the height in the tube. a. Radius = 0.1 mm, 0.5 mm, 1 mm, 2 mm (the slide bar is often difficult to get exact values - record your values) 3. Set the radius to be 1 mm and measure the height of the remaining liquids in the list above using the specific gravity and surface tension given. 4. Use your observations to predict the types of intermolecular forces present in each liquid. Confirm these with Lewis structures, electronegativity, and VSEPR theory. 5. List (in a table) the height (h) values for the liquids and diameter of the capillary tube and then calculate the capillary force for each of the liquids. The formula that relates the capillary force, fc to other properties is given in the following equation, 1. = mrdgh d=density Where force, is in Newtons (kgm/s"); r = radius of the capillary tube (in cm); d = density of the liquid (in g/cm', which is equivalent to g/mL); g = gravitational force (9.8 m/s); and h = height of the liquid in the capillary Show the calculation of Capillary Force for water and ethylene glycol. 6. Place a few drops of each of the following liquids in a well plate. A: water B: ethylene glycol C: hewane em D: ethanol ve e Methanol con 7. Place a capillary tube in each of the liquids. Hold it vertical but do not cover the top end with your finger 8. Record whether the liquid has a concave, flat or convex meniscus in each capillary tube. 9. After the liquid's height has stabilized, mark the level of the liquid in the well and the height of the liquid in the capillary tube with a marker. 10. Remove the capillary tube from the liquid and use a ruler to measure the height (h) of the liquid in the capillary tube. 11. Record the inside diameter of the capillary tube. . Otam 9 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


