Answered step by step
Verified Expert Solution
Question
1 Approved Answer
How to compare the constant a from the trendline equation with theoretical values? Given by professor: Trendline p=aV^b Theoretical p=NKT *V^-1. Compare a with NkT.
How to compare the constant "a" from the trendline equation with theoretical values? Given by professor: Trendline p=aV^b Theoretical p=NKT *V^-1. Compare "a" with NkT. All data are provided. No missing information
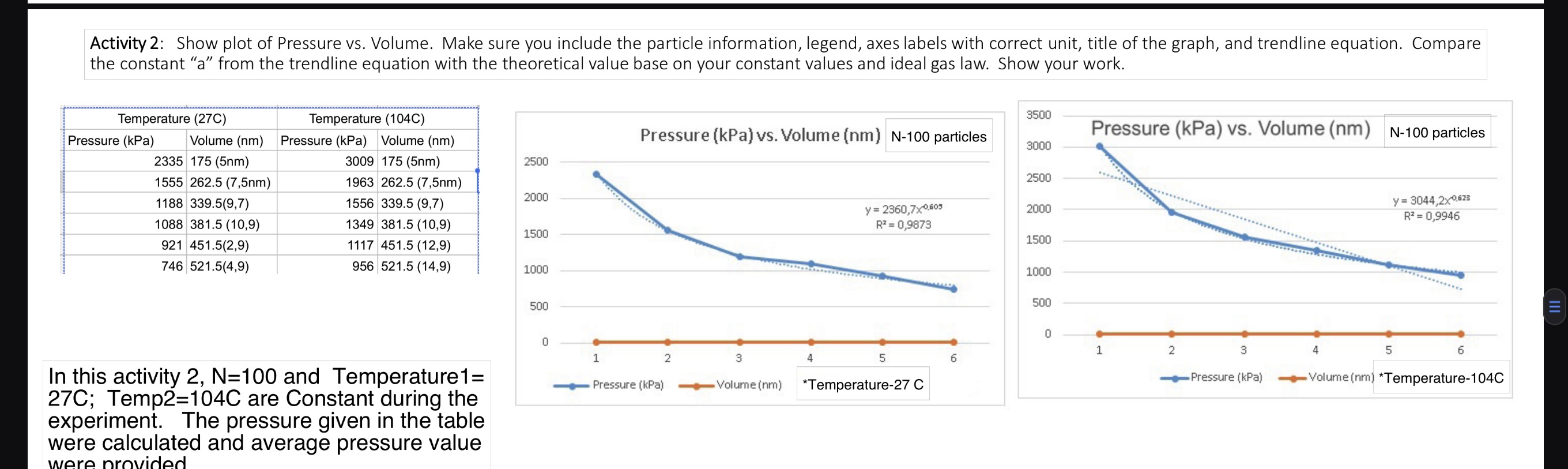


Activity 2: Show plot of Pressure vs. Volume. Make sure you include the particle information, legend, axes labels with correct unit, title of the graph, and trendline equation. Compare the constant "a" from the trendline equation with the theoretical value base on your constant values and ideal gas law. Show your work. 3500 Temperature (27C) Temperature (104C) Pressure (kPa) Volume (nm) 2335 175 (5nm) 1555 262.5 (7,5nm) Pressure (kPa) Volume (nm) 3009 175 (5nm) Pressure (kPa) vs. Volume (nm) N-100 particles Pressure (kPa) vs. Volume (nm) N-100 particles 3000 2500 2500 1963 262.5 (7,5nm) 2000 1188 339.5(9,7) 1556 339.5 (9,7) 1088 381.5 (10,9) 1349 381.5 (10,9) 1500 y = 2360,7x0,605 2000 R = 0,9873 1500 y= 3044,2x0,623 R = 0,9946 921 451.5(2,9) 1117 451.5 (12,9) 746 521.5(4,9) 956 521.5 (14,9) 1000 1000 In this activity 2, N=100 and Temperature1= 27C; Temp2=104C are Constant during the experiment. The pressure given in the table were calculated and average pressure value were provided 500 0 1 2 3 4 Pressure (kPa) Volume (nm) 5 *Temperature-27 C 500 0 1 2 3 4 6 Pressure (kPa) 5 6 Volume (nm) *Temperature-104C
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


