Question
How to determine the exit gas density in Q2: Ideal gas law PV=nRT Density: p = ,V = mass Number of moles: n =
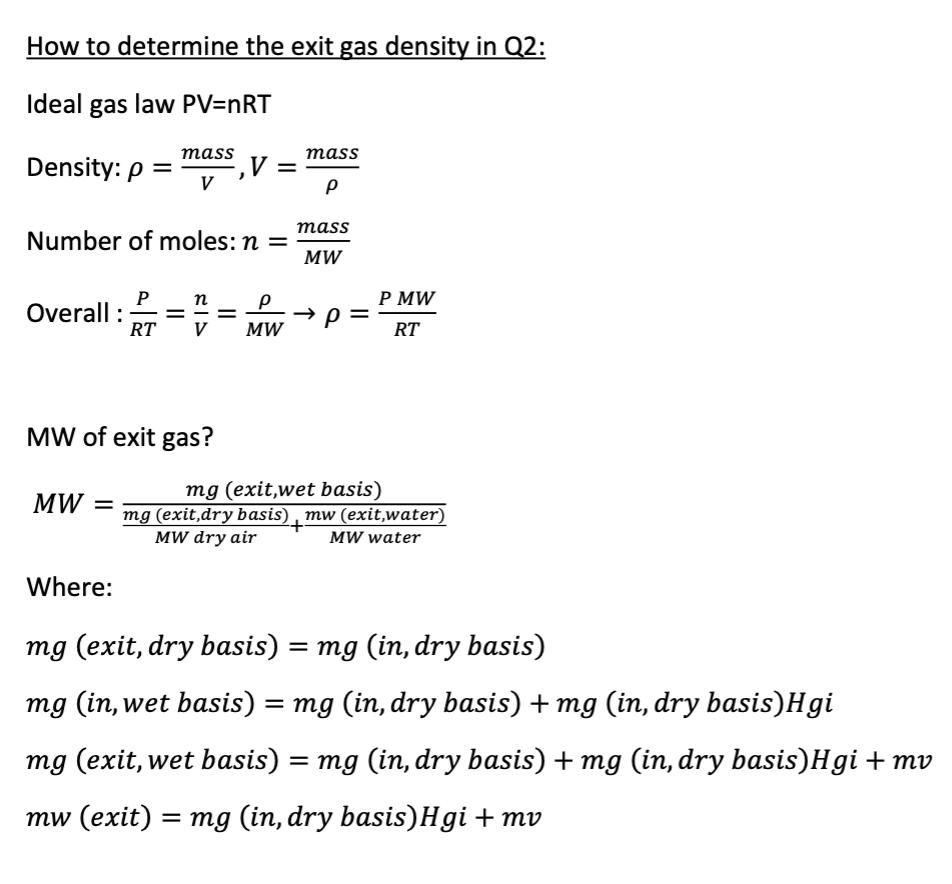
How to determine the exit gas density in Q2: Ideal gas law PV=nRT Density: p = ,V = mass Number of moles: n = P n P Overall: RT V MW MW of exit gas? MW Where: mass P mass MW PMW RT mg (exit,wet basis) mg (exit,dry basis) mw (exit,water) MW dry air MW water mg (exit, dry basis) = mg (in, dry basis) mg (in, wet basis) = mg (in, dry basis) + mg (in, dry basis)Hgi mg (exit, wet basis) = mg (in, dry basis) + mg (in, dry basis)Hgi+mv mw (exit) = = mg (in, dry basis) Hgi + mv
Step by Step Solution
3.43 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1
To determine the exit gas density we need to use the ideal gas law PV nRT First we need to find the ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Numerical Methods With Chemical Engineering Applications
Authors: Kevin D. Dorfman, Prodromos Daoutidis
1st Edition
1107135117, 978-1107135116
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



