Answered step by step
Verified Expert Solution
Question
1 Approved Answer
How to prepare 100 mL of 10ppm of Cu2+ and Zn2+ in step 12 and 13 In this experiment, a penny is dissolved in nitric
How to prepare 100 mL of 10ppm of Cu2+ and Zn2+ in step 12 and 13 
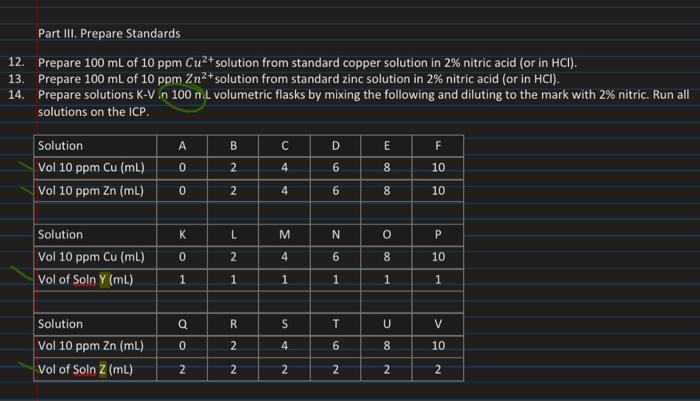
In this experiment, a penny is dissolved in nitric acid, HNO; (reactions shown below). The redox reaction shown below (M = Cu, Zn) occurs to generate copper(II) and zinc(ll) ions. M(s) + 4HNO, (aq) + 4H,0(1) - MCH,0)3+ (aq) + 2N0;(9) + 2NO3(aq) The result is a highly acidic solution containing the analytes, Cu and Zn. This solution needs to be diluted for use on the ICP instrument. Using a standard addition method, the concentrations of the copper and zinc ions will be found, and these values will be used to determine the weight percent of each element in a penny, a Part III. Prepare Standards 12. Prepare 100 mL of 10 ppm Cu2+ solution from standard copper solution in 2% nitric acid (or in HCI). 13. Prepare 100 mL of 10 ppm Zn2+ solution from standard zinc solution in 2% nitric acid (or in HCI). 14. Prepare solutions K-V .n 100 nil volumetric flasks by mixing the following and diluting to the mark with 2% nitric. Run all solutions on the ICP. B C D E F Solution Vol 10 ppm Cu (mL) Vol 10 ppm Zn (ML) 0 2 4 6 LO 00 00 m 8 10 o N 4 6 10 K L M N o P Solution Vol 10 ppm Cu (mL) Vol of Soln Y (ml) o Z 0 2 4 6 8 10 1 1 1 1 1 Q R S T U > Solution Vol 10 ppm Zn (mL) Vol of Soln Z (ml) do N 0 2 4 6 N 00 C 8 10 2 2 N 2 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


