Answered step by step
Verified Expert Solution
Question
1 Approved Answer
How would you be able to get these values? For a step polymerization of monomers, ARA (with M0,A=90g/mole ) and BRB (with M0,B =140g/ mole),
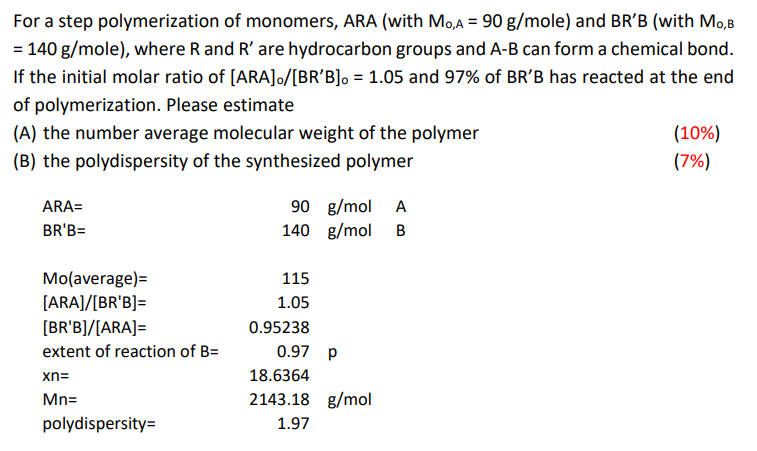
How would you be able to get these values?
For a step polymerization of monomers, ARA (with M0,A=90g/mole ) and BRB (with M0,B =140g/ mole), where R and R are hydrocarbon groups and AB can form a chemical bond. If the initial molar ratio of [ARA]/[BRB]=1.05 and 97% of BRB has reacted at the end of polymerization. Please estimate (A) the number average molecular weight of the polymer (10%) (B) the polydispersity of the synthesized polymer (7%)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


