Answered step by step
Verified Expert Solution
Question
1 Approved Answer
6-6 Lead melts at 620F and tin melts at 450F. They form a eutectic containing 62 percent tin at 360F. The maximum solid solubility
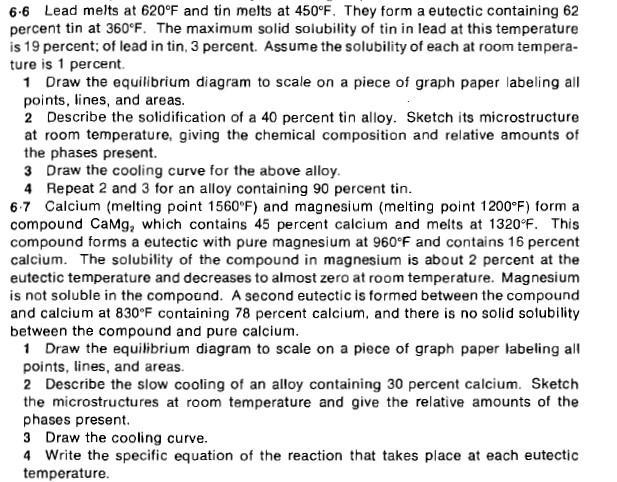
6-6 Lead melts at 620F and tin melts at 450F. They form a eutectic containing 62 percent tin at 360F. The maximum solid solubility of tin in lead at this temperature is 19 percent; of lead in tin, 3 percent. Assume the solubility of each at room tempera- ture is 1 percent. 1 Draw the equilibrium diagram to scale on a piece of graph paper labeling all points, lines, and areas. 2 Describe the solidification of a 40 percent tin alloy. Sketch its microstructure at room temperature, giving the chemical composition and relative amounts of the phases present. 3 Draw the cooling curve for the above alloy. 4 Repeat 2 and 3 for an alloy containing 90 percent tin. 6-7 Calcium (melting point 1560F) and magnesium (melting point 1200F) form a compound CaMg, which contains 45 percent calcium and melts at 1320F. This compound forms a eutectic with pure magnesium at 960F and contains 16 percent calcium. The solubility of the compound in magnesium is about 2 percent at the eutectic temperature and decreases to almost zero at room temperature. Magnesium is not soluble in the compound. A second eutectic is formed between the compound and calcium at 830F containing 78 percent calcium, and there is no solid solubility between the compound and pure calcium. 1 Draw the equilibrium diagram to scale on a piece of graph paper labeling all points, lines, and areas. 2 Describe the slow cooling of an alloy containing 30 percent calcium. Sketch the microstructures at room temperature and give the relative amounts of the phases present. 3 Draw the cooling curve. 4 Write the specific equation of the reaction that takes place at each eutectic temperature.
Step by Step Solution
★★★★★
3.39 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


