Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Bakers' yeast is produced in a 50,000 litre fermenter under aerobic conditions. The carbon substrate is sucrose; ammonia is provided as the nitrogen source.
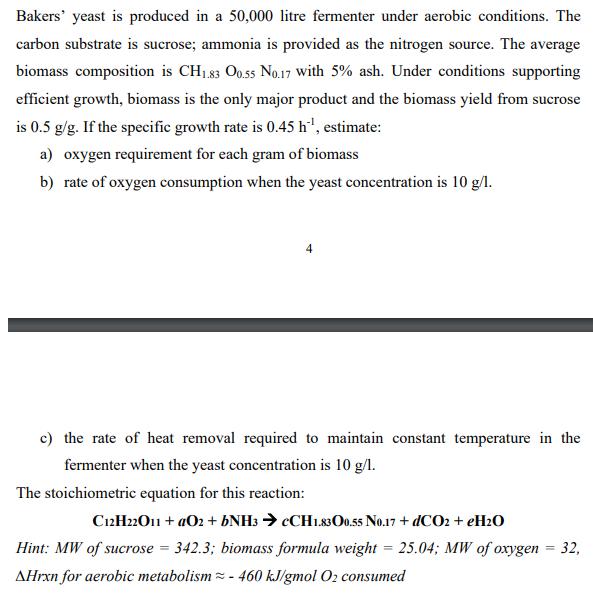
Bakers' yeast is produced in a 50,000 litre fermenter under aerobic conditions. The carbon substrate is sucrose; ammonia is provided as the nitrogen source. The average biomass composition is CH183 O0.5s No.17 with 5% ash. Under conditions supporting efficient growth, biomass is the only major product and the biomass yield from sucrose is 0.5 g/g. If the specific growth rate is 0.45 h', estimate: a) oxygen requirement for each gram of biomass b) rate of oxygen consumption when the yeast concentration is 10 g/l. 4 c) the rate of heat removal required to maintain constant temperature in the fermenter when the yeast concentration is 10 g/l. The stoichiometric equation for this reaction: C12H22O11 + aO2 + 6NH3 > CCH1.83O0.55 No.17 + dCO2 + EH2O Hint: MW of sucrose = 342.3; biomass formula weight = 25.04; MW of oxygen 32, AHrxn for aerobic metabolism = - 460 kJ/gmol O2 consumed
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
6092dc1737512_210548.pdf
180 KBs PDF File
6092dc1737512_210548.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


