A gas phase reaction between butadiene and ethylene is conducted in a PFR, producing cyclohexene: C4H6(g) + C2H4(g) >C6H10(g) + A2 (A1 A3) ->
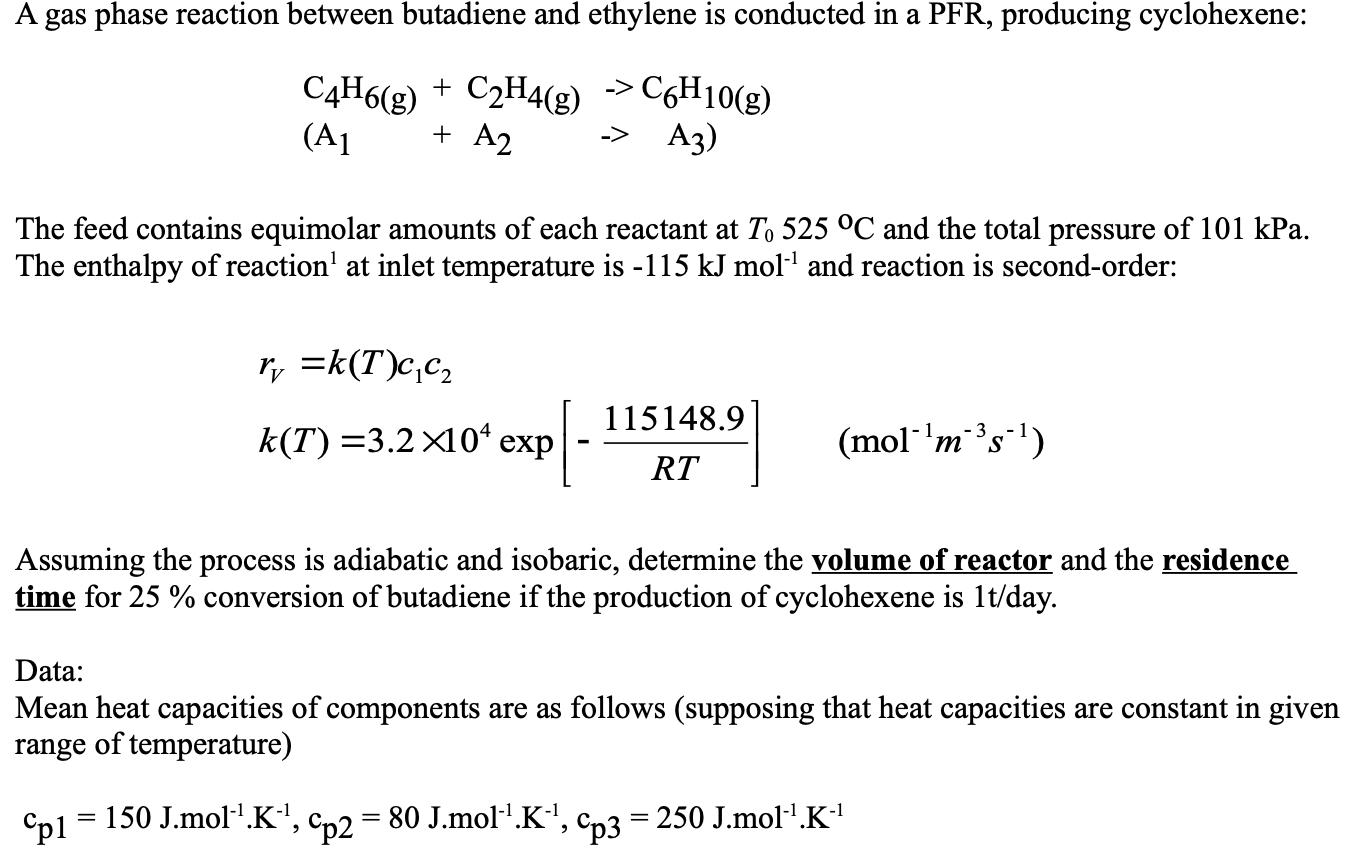
A gas phase reaction between butadiene and ethylene is conducted in a PFR, producing cyclohexene: C4H6(g) + C2H4(g) >C6H10(g) + A2 (A1 A3) -> The feed contains equimolar amounts of each reactant at To 525 C and the total pressure of 101 kPa. The enthalpy of reaction' at inlet temperature is -115 kJ mol' and reaction is second-order: ry =k(T)c,c, 115148.9 k(T) =3.2 X10* exp (mol-'ms) RT Assuming the process is adiabatic and isobaric, determine the volume of reactor and the residence time for 25 % conversion of butadiene if the production of cyclohexene is 1t/day. Data: Mean heat capacities of components are as follows (supposing that heat capacities are constant in given range of temperature) "pl 150 J.mol'.K', Cp2 80 J.mol1.K', Cp3 = 250 J.mol-.K
Step by Step Solution
There are 3 Steps involved in it
Step: 1

See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


