Question
Hydrogen (H2) reacts with 400% theoretical air in an isothermal reaction where the initial and final conditions are 25 and 1 atm pressure. The surroundings
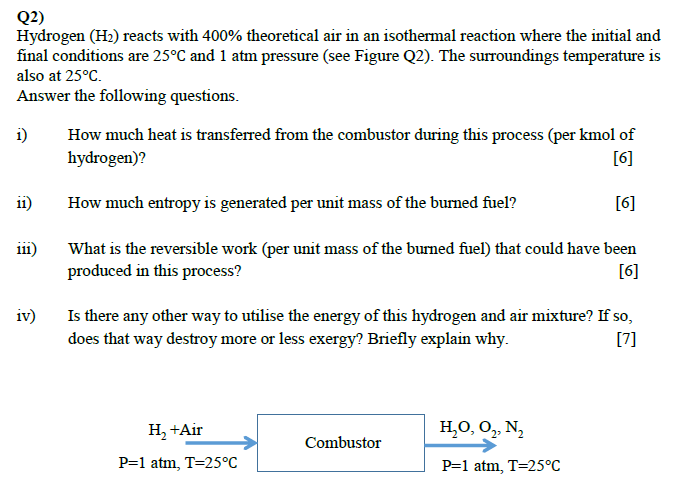
Hydrogen (H2) reacts with 400% theoretical air in an isothermal reaction where the initial and final conditions are 25 and 1 atm pressure. The surroundings temperature is also at 25. Answer the following questions. i) How much heat is transferred from the combustor during this process (per kmol of hydrogen)? ii) How much entropy is generated per unit mass of the burned fuel? iii) What is the reversible work (per unit mass of the burned fuel) that could have been produced in this process? iv) Is there any other way to utilise the energy of this hydrogen and air mixture? If so, does that way destroy more or less exergy? Briefly explain why.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


