Question
Hydrogen, important for numerous processes, can be produced by the shift reaction: CO+ H20CO2+H2 In the reactor system shown in Figure P12.15, the conditions
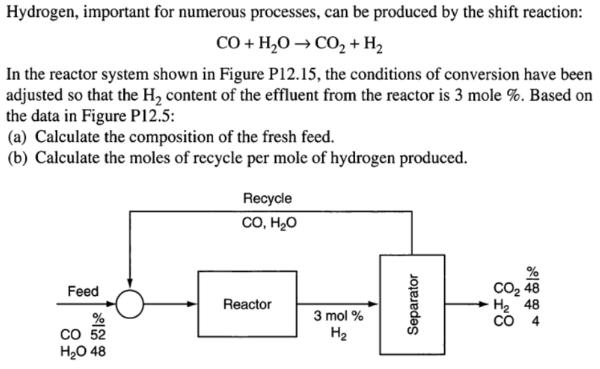
Hydrogen, important for numerous processes, can be produced by the shift reaction: CO+ H20CO2+H2 In the reactor system shown in Figure P12.15, the conditions of conversion have been adjusted so that the H2 content of the effluent from the reactor is 3 mole %. Based on the data in Figure Pl12.5: (a) Calculate the composition of the fresh feed. (b) Calculate the moles of recycle per mole of hydrogen produced Recycle , , CO2 48 H2 48 CO 4 Feed Reactor 3 mol % % CO 52 48 H2 Separator
Step by Step Solution
3.42 Rating (149 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Introduction to Operations Research
Authors: Frederick S. Hillier, Gerald J. Lieberman
10th edition
978-0072535105, 72535105, 978-1259162985
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



