Answered step by step
Verified Expert Solution
Question
1 Approved Answer
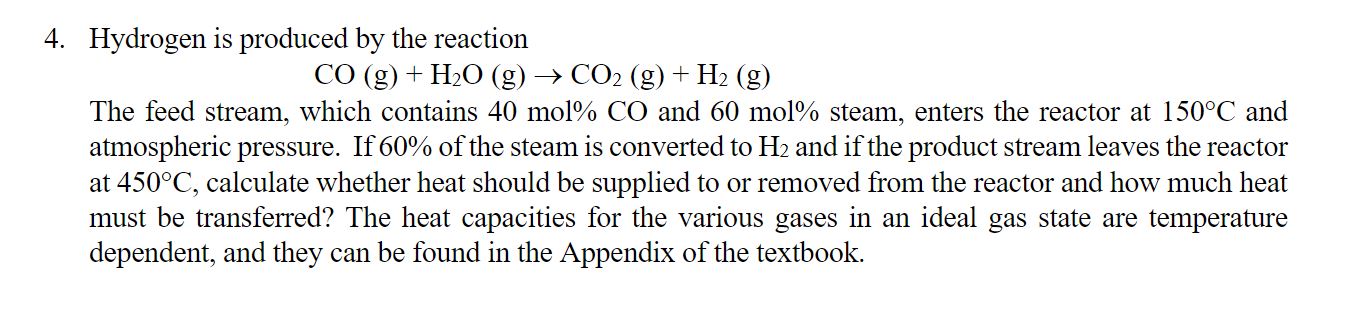
Hydrogen is produced by the reaction C O ( g ) + H 2 O ( g ) C O 2 ( g ) +
Hydrogen is produced by the reaction
The feed stream, which contains mol and mol steam, enters the reactor at and
atmospheric pressure. If of the steam is converted to and if the product stream leaves the reactor
at calculate whether heat should be supplied to or removed from the reactor and how much heat
must be transferred? The heat capacities for the various gases in an ideal gas state are temperature
dependent, and they can be found in the Appendix of the textbook.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


