Question
Hydrogen peroxide decomposes to water and oxygen by the following reaction: H2O2 --------> H20 + O2 At 40C, the following data was taken for
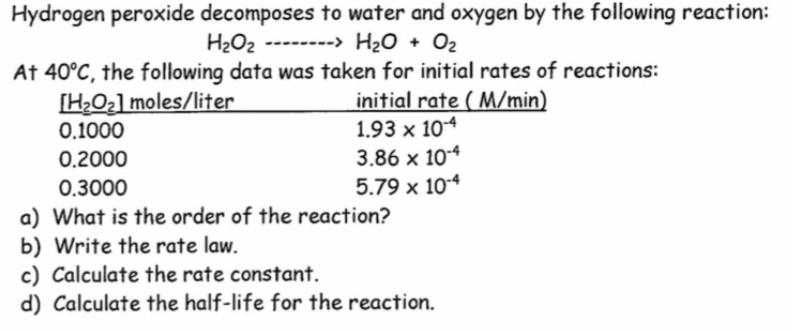
Hydrogen peroxide decomposes to water and oxygen by the following reaction: H2O2 --------> H20 + O2 At 40C, the following data was taken for initial rates of reactions: [HO2] moles/liter initial rate ( M/min) 1.93 x 104 3.86 x 104 5.79 x 10-4 0.1000 0.2000 0.3000 a) What is the order of the reaction? b) Write the rate law. c) Calculate the rate constant. d) Calculate the half-life for the reaction.
Step by Step Solution
3.34 Rating (160 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
General Chemistry
Authors: Darrell Ebbing, Steven D. Gammon
9th edition
978-0618857487, 618857486, 143904399X , 978-1439043998
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



