Answered step by step
Verified Expert Solution
Question
1 Approved Answer
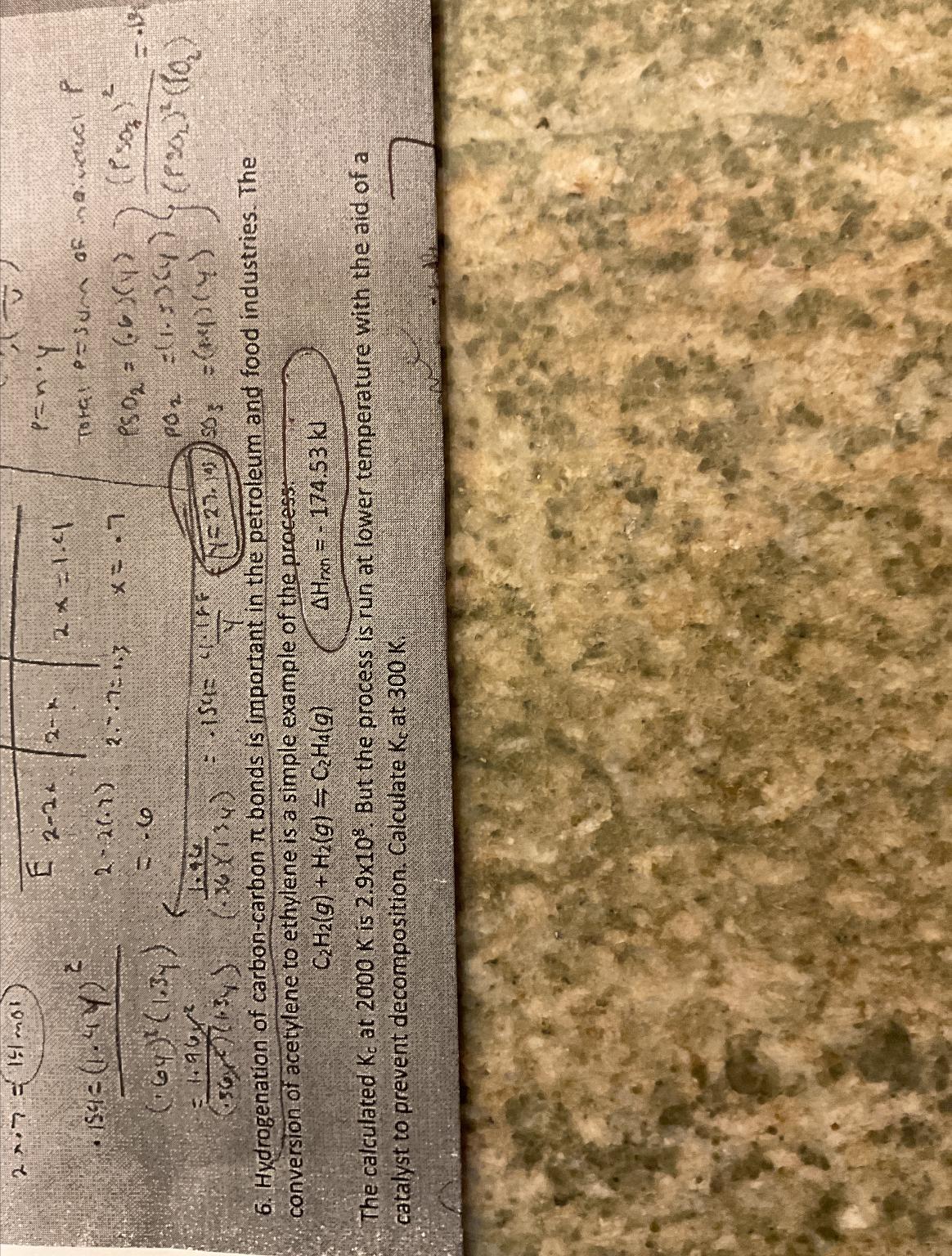
Hydrogenation of carbon-carbon pi bonds is important in the petroleum and food industries. The conversion of acetylene to ethylene is a simple example of the
Hydrogenation of carbon-carbon
\\\\pi bonds is important in the petroleum and food industries. The conversion of acetylene to ethylene is a simple example of the process:\
C_(2)H_(2)(g)+H_(2)(g)C_(2)H_(4)(g),\\\\Delta H_(rn )=-174.53kJ\ The calculated
K_(c)at
2000Kis
2.9\\\\times 10^(8). But the process is run at lower temperature with the aid of a catalyst to prevent decomposition. Calculate
K_(c)at
300K.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


