Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(i) A sample of coal contains C = 89%; H = 9% and ash = 2%. The following data were obtained when the above coal
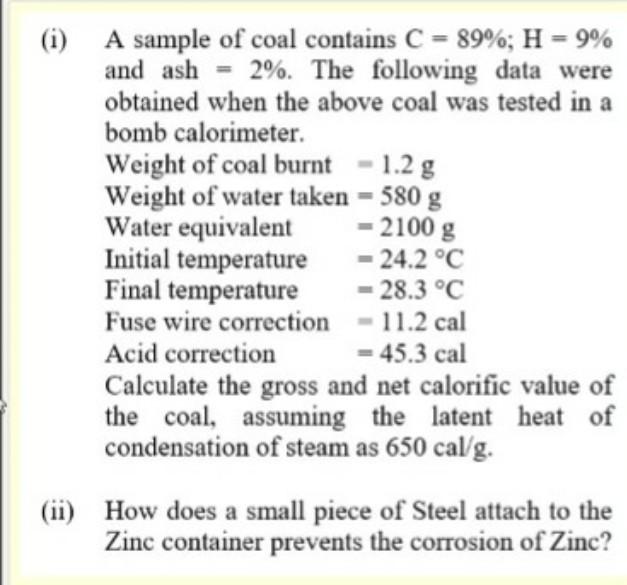
(i) A sample of coal contains C = 89%; H = 9% and ash = 2%. The following data were obtained when the above coal was tested in a bomb calorimeter. Weight of coal burnt - 1.2 g Weight of water taken = 580 g Water equivalent = 2100 g Initial temperature - 24.2 C Final temperature 28.3 C Fuse wire correction 11.2 cal Acid correction = 45.3 cal Calculate the gross and net calorific value of the coal, assuming the latent heat of condensation of steam as 650 cal/g. (ii) How does a small piece of Steel attach to the Zinc container prevents the corrosion of Zinc
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


