Question
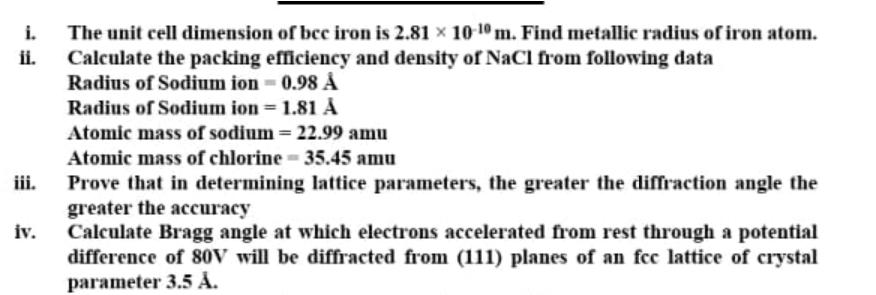
i. Calculate the packing efficiency and density of NaCl from following data Radius of Sodium ion 0.98 The unit cell dimension of bce iron
i. Calculate the packing efficiency and density of NaCl from following data Radius of Sodium ion 0.98 The unit cell dimension of bce iron is 2.81 x 10 10 m. Find metallic radius of iron atom. ii. Radius of Sodium ion = 1.81 %3D Atomic mass of sodium = 22.99 amu Atomic mass of chlorine 35.45 amu iii. Prove that in determining lattice parameters, the greater the diffraction angle the greater the accuracy iv. Calculate Bragg angle at which electrons accelerated from rest through a potential difference of 8oV will be diffracted from (111) planes of an fcc lattice of crystal parameter 3.5 .
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry
Authors: Raymond Chang
10th edition
77274318, 978-0077274313
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App