Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I can't for the life of mefigure this out Complete the following sentences about strong and weak acids and bases. Ca(OH) HNO, LiOH CHCOOH oxoacids
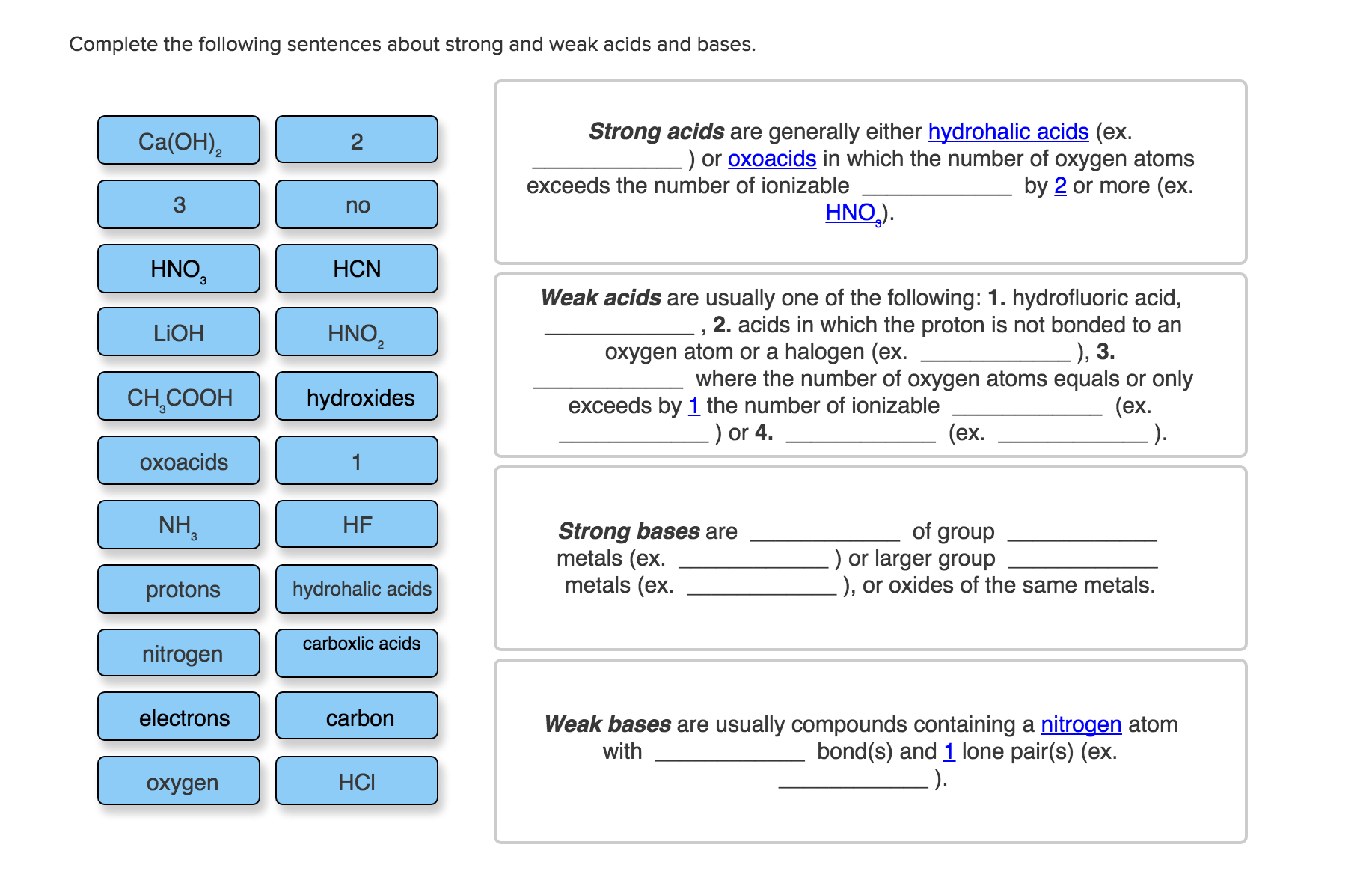 I can't for the life of mefigure this out
I can't for the life of mefigure this out
Complete the following sentences about strong and weak acids and bases. Ca(OH) HNO, LiOH CHCOOH oxoacids NH protons nitrogen electrons oxygen 2 no HCN HNO hydroxides HF hydrohalic acids carboxlic acids carbon HCI Strong acids are generally either hydrohalic acids (ex. .) or oxoacids in which the number of oxygen atoms exceeds the number of ionizable by 2 or more (ex. HNO). Weak acids are usually one of the following: 1. hydrofluoric acid, 2 2. acids in which the proton is not bonded to an oxygen atom or a halogen (ex. ), 3. where the number of oxygen atoms equals or only exceeds by 1 the number of ionizable (ex. .) or 4. (ex. Strong bases are metals (ex. metals (ex. of group .) or larger group ), or oxides of the same metals. Weak bases are usually compounds containing a nitrogen atom bond(s) and 1 lone pair(s) (ex. .). with
Step by Step Solution
★★★★★
3.51 Rating (154 Votes )
There are 3 Steps involved in it
Step: 1
Strong acids are generally either hydrohalic acids exHCI or oxoaci...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


