Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I. Chemistry: A. Consider the chemical reaction: PbN6+CrMn2O8Pb3O4+Cr2O3+MnO2+NO a. Let a,b,c,d,ef denote the number of molecules of each type that are needed to balance the
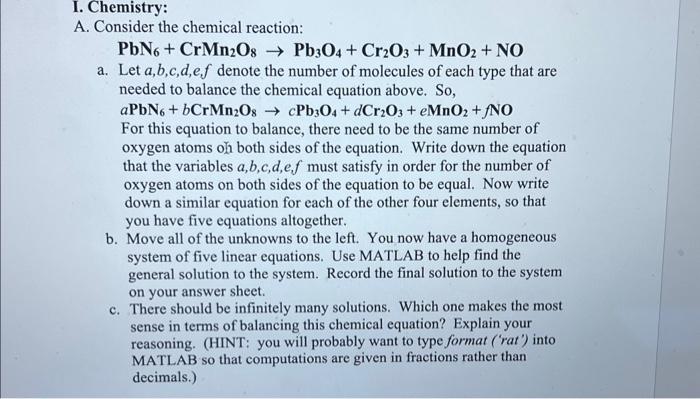 I. Chemistry: A. Consider the chemical reaction: PbN6+CrMn2O8Pb3O4+Cr2O3+MnO2+NO a. Let a,b,c,d,ef denote the number of molecules of each type that are needed to balance the chemical equation above. So, aPbN6+bCrMn2O8cPb3O4+dCr2O3+eMnO2+fNO2 For this equation to balance, there need to be the same number of oxygen atoms ohi both sides of the equation. Write down the equation that the variables a,b,c,d,e,f must satisfy in order for the number of oxygen atoms on both sides of the equation to be equal. Now write down a similar equation for each of the other four elements, so that you have five equations altogether. b. Move all of the unknowns to the left. You now have a homogeneous system of five linear equations. Use MATLAB to help find the general solution to the system. Record the final solution to the system on your answer sheet. c. There should be infinitely many solutions. Which one makes the most sense in terms of balancing this chemical equation? Explain your reasoning. (HINT: you will probably want to type format ('rat') into MATLAB so that computations are given in fractions rather than decimals.)
I. Chemistry: A. Consider the chemical reaction: PbN6+CrMn2O8Pb3O4+Cr2O3+MnO2+NO a. Let a,b,c,d,ef denote the number of molecules of each type that are needed to balance the chemical equation above. So, aPbN6+bCrMn2O8cPb3O4+dCr2O3+eMnO2+fNO2 For this equation to balance, there need to be the same number of oxygen atoms ohi both sides of the equation. Write down the equation that the variables a,b,c,d,e,f must satisfy in order for the number of oxygen atoms on both sides of the equation to be equal. Now write down a similar equation for each of the other four elements, so that you have five equations altogether. b. Move all of the unknowns to the left. You now have a homogeneous system of five linear equations. Use MATLAB to help find the general solution to the system. Record the final solution to the system on your answer sheet. c. There should be infinitely many solutions. Which one makes the most sense in terms of balancing this chemical equation? Explain your reasoning. (HINT: you will probably want to type format ('rat') into MATLAB so that computations are given in fractions rather than decimals.)

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


