Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I do not want the change in standard reaction entropy. I want the overall change in entropy given my change in pressure, and the fact
I do not want the change in standard reaction entropy. I want the overall change in entropy given my change in pressure, and the fact my temperature is the same (standard temp is 25C, and the given temp is 25C). This process is done in a bomb calorimeter, does this also mean that volume is constant?
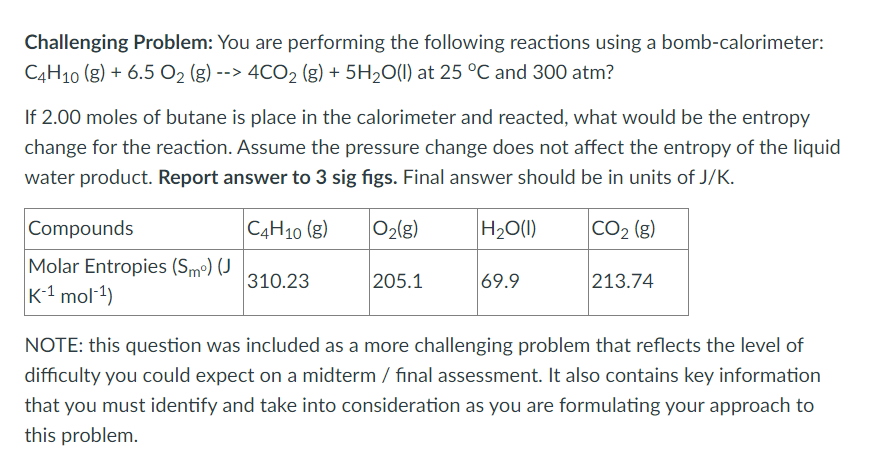
Challenging Problem: You are performing the following reactions using a bomb-calorimeter: C4H10(g)+6.5O2(g)>4CO2(g)+5H2O(l) at 25C and 300 atm? If 2.00 moles of butane is place in the calorimeter and reacted, what would be the entropy change for the reaction. Assume the pressure change does not affect the entropy of the liquid water product. Report answer to 3 sig figs. Final answer should be in units of J/K. NOTE: this question was included as a more challenging problem that reflects the level of difficulty you could expect on a midterm / final assessment. It also contains key information that you must identify and take into consideration as you are formulating your approach to thisStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


