Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I filled out as much as i can, pls solve the column for Psyr(Pa) and the two that follow it. USING THE DATA IN RED!!
I filled out as much as i can, pls solve the column for Psyr(Pa) and the two that follow it. USING THE DATA IN RED!! 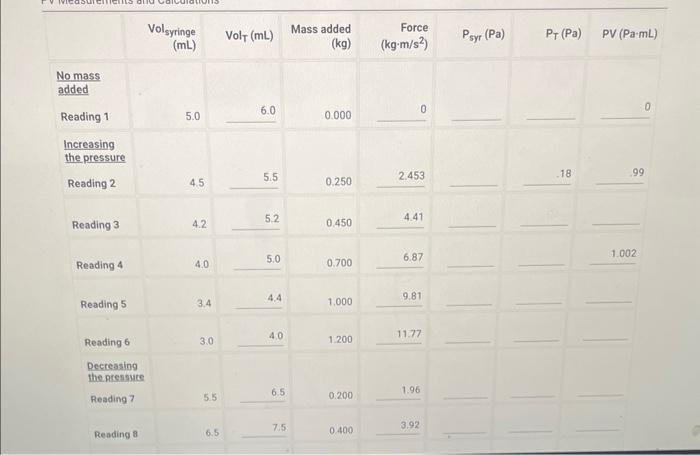
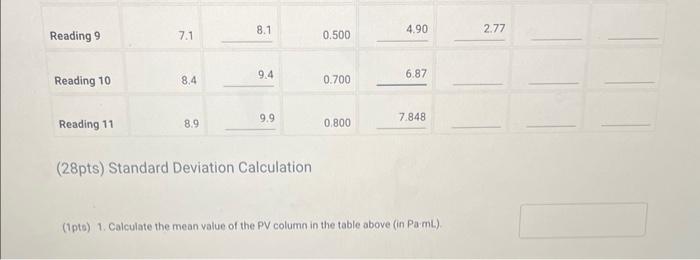
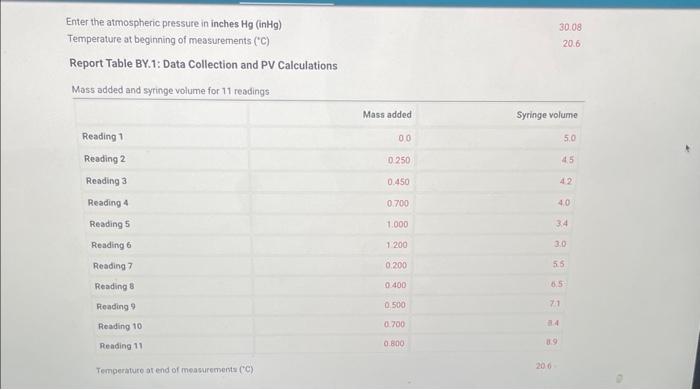
No mass added Reading 1 5.0 0.000 Increasing the pressure Reading 2 \begin{tabular}{llll} 4.5 & 5.5 & 0.250 & 2.453 \\ \hline \end{tabular} Reading 3 \begin{tabular}{llll} 4.2 & 5.2 & 0.450 & 4.41 \\ \hline \end{tabular} Reading 4 4.0 5.0 0.7006.87 \begin{tabular}{llll} 3.4 & 4.4 & 1.000 & 9.81 \\ \hline \end{tabular} Reading 6 3.0 4.0 1.20011.77 Decreasing the pressute Reading 7 \begin{tabular}{lll} 5.5 & 6.5 & 0.2001.96 \\ \hline 6.5 & 7.5 & 0.4003.92 \\ \hline \end{tabular} (28pts) Standard Deviation Calculation (1pto) 1. Calculate the mean value of the PV column in the table above (in Pa mL ). Enter the atmospheric pressure in inches Hg(inHg) Temperature at beginning of measurements ('C) Report Table BY.1: Data Collection and PV Calculations 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


