Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I found to Solutions one of them the answer is Kp=4.75 and Kp=367.18 I do not know which one is correct s. The equilibrium condition
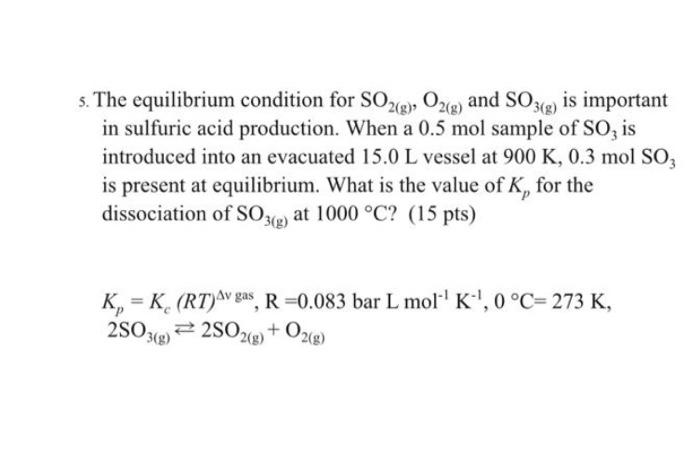
I found to Solutions one of them the answer is Kp=4.75 and Kp=367.18 I do not know which one is correct
s. The equilibrium condition for SO2(g), O2(g) and SO3(g) is important in sulfuric acid production. When a 0.5 mol sample of SO, is introduced into an evacuated 15.0 L vessel at 900 K, 0.3 mol SO, is present at equilibrium. What is the value of K, for the dissociation of SO3(g) at 1000 C? (15 pts) K, = K (RT)Avgas, R =0.083 bar L mol-'K',0C=273 K, 25039 = 2502(g) + O2(8) Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


