i just need the best and correct answer this question to help me prepare for the lab exam coming up. Thank you so much for helping me.
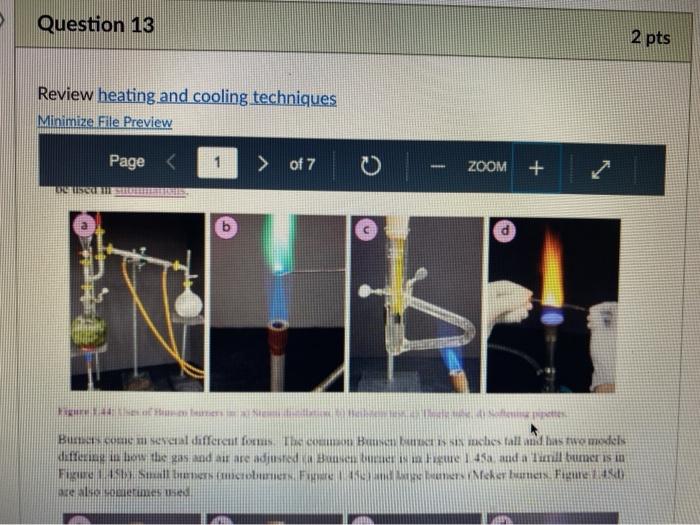
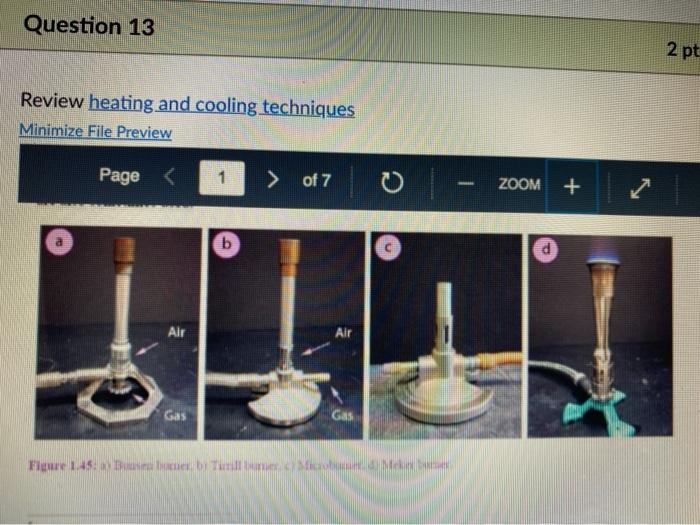
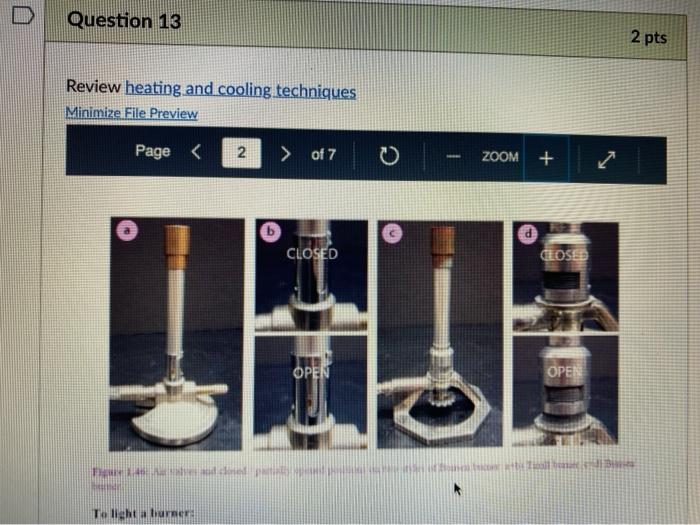
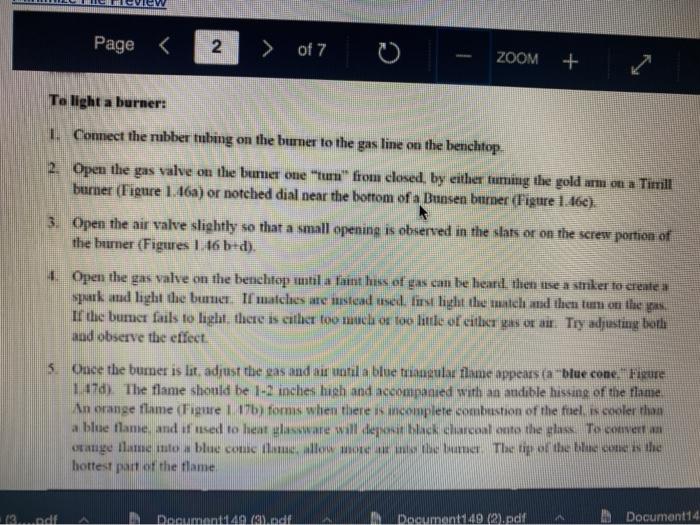
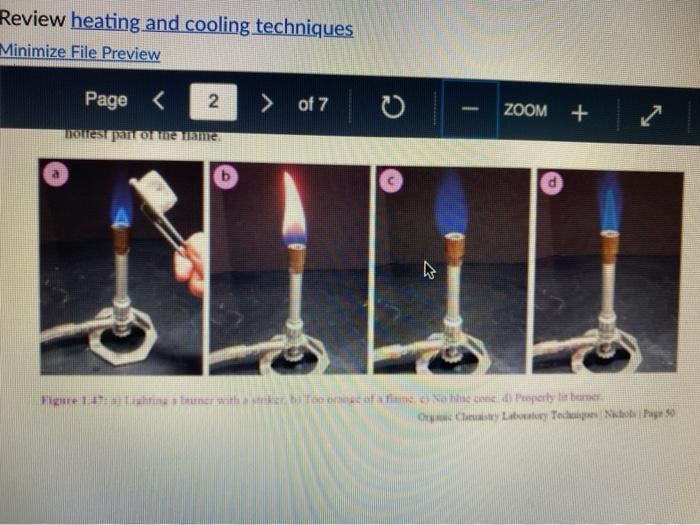
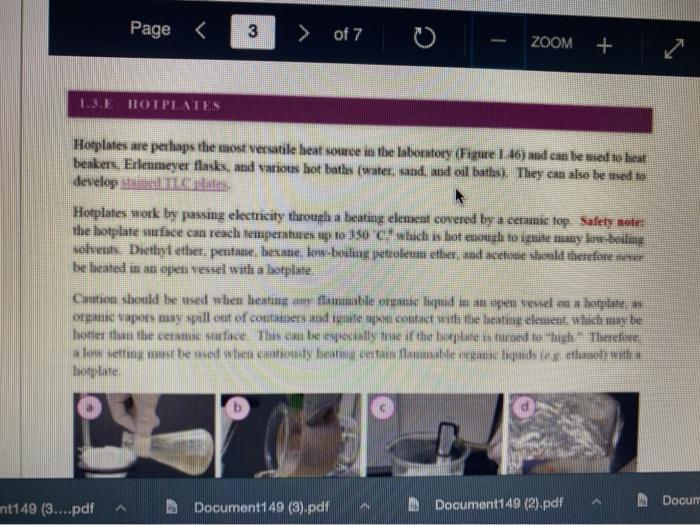
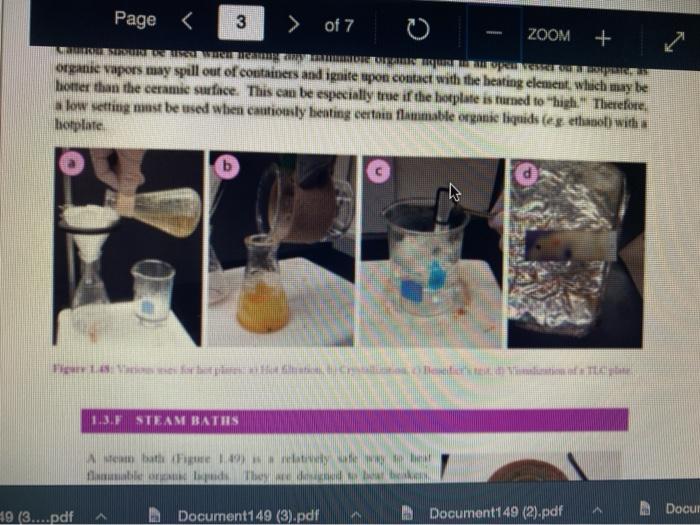
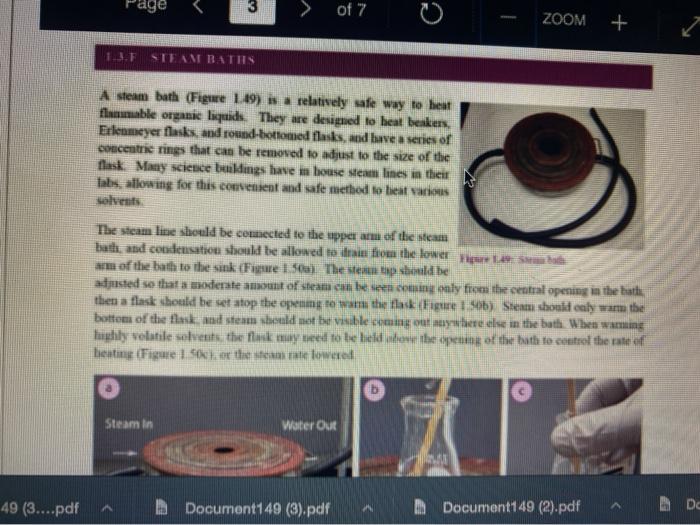
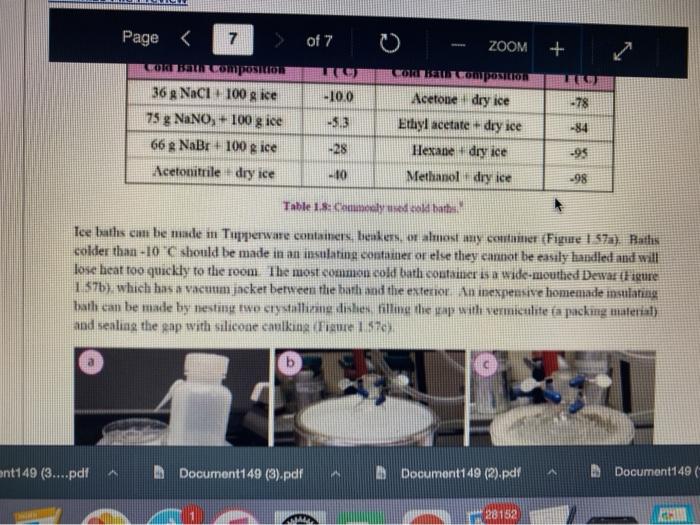
Question 13 2 pts Review heating and cooling techniques Minimize File Preview Page > of 7 ZOOM + 1.3.D BUNSEN BURNERS Buasen burgers are generally used to rapidly beat high-boiling liquids with low flammability such as water). Safety notes tris important to know that they can reach temperatures of approximately 1500 C and can easily ignite most organic compounds. If na apparatus as improperly set up, or if there is a small sap that allows organe vapors to escape from an apparatus, these vapors can ignite with a buner Therefore, it is renerally recommended to the other liest ces to warm tasumable and liquid (for example or) Bunsen burners should never be red with highly flimable solvents such as diety ethet However, bunes de liave the place in the organica Husebiotisci in (Figure tas as the vapors are generally not tamil Ini di contest wie eiset stop a ring copiolet ned tider the flask to dissipate dhe e ndavad necesare Burners are bored in the for balogan (Figu waliwahi de 100 id for More Hirine 1 Aldi Thiey INI Question 13 2 pts Review heating and cooling techniques Minimize File Preview Page of 7 0 - ZOOM + TUTURE Het is een tes Bu, come several different ts Te common Busen HS sis melies tall and bas two models daterem bow the gas and an are adjusted Buseu bene in me 1 45a, and a Tamil bumer is in Figure Stall bene cioburners Free beamers Nicher lumen Figure 1.430 Genes Question 13 2 pt Review heating and cooling techniques Minimize File Preview Page of 7 0 ZOOM + b Air Air Gas Gas Fire 1.0 Bibe bunlar Neck Question 13 2 pts Review heating and cooling techniques Minimize File Preview Page of 7 ZOOM + CLOSED DEOSED OPEN OPEN Add como Tellet a burner Page of 7 ZOOM + To light a burner: 1. Connect the rubber tubing on the bener to the gas line on the benchtop. 2. Open the gas valve on the bumer one from closed, by either tuming the gold am ou a Tamil burner (Figure 1.46a) or notched dial near the bottom of a Bunsen bumer (Figure 1.46). 3. Open the air valve slightly so that a small opening is observed in the slats or on the screw portion of the banner (Figures 1.46 b+d). 1. Open the gas valve on the benchtop itil a faini hiss of gas can be heard then use a striker to create a uukaud light the bume. If matches are stead used in light the mustel and then tum om te gas If the bumer fails to light, there is either too much or too little of either was orau. Try adjusting both and observe the effect. 5. Once the bumer is lit. adjust the gas and an until a blue triangular flame appears (a "blue cone. Figure 1170). The flame should be 10 inches high and accompanied with an audible hissing of the flame in orange flame (Figure 1 176forms when there is complete combustion of the fuel is cooler thun a blue flame and if used to heat glassware will get back curcoal onto the glas. Te convert orange flame ute a blue come flame allow more in the bummerThe tip of the blue cues the hottest part of the flame (2...ndt Documentada Document 149 2).pdf Document14 Review heating and cooling techniques Minimize File Preview Page of 7 ZOOM + Totesto etme, orain aukera med Property it bummer Chwy Laboratory Ted Nution Pay 50 Page of 7 ZOOM + CURSURI NURULARI uR organie vapors may spill out of containers and ignite por contact with the heating element, which may be hotter than the ceramic surface. This can be especially true if the hotplate is turned to "high. There a low setting must be used when cautiously henting certain flammable organic liquids (eg ethol) with hotplate b 1.F STEAM BATIS AMOR bath ( Ferry namun di The door Docur 19 (3....pdf Document149 (3).pdf Document149 (2).pdf Page 3 of 2 C ZOOM + STEMRATIS A steam bath (Figure 1.19) is a relatively safe way to heat flammable organic liquids. They are designed to heat beakers, Erlenmeyer flasks and round-bottomed flasks, and have a series of concentric rings that can be removed to adjust to the size of the task. Many science buildings have a house sterne lines in their tabs, allowing for this convenient and safe method to beat various The steam line should be commected to the upper arm of the steam batt, and condensatiou should be allowed to desia thon the lower the Sembole m of the bath to the sink (Figure 1 500 The stenu tapi skuld be usted so that a moderate amit of steam can be seen coming only from the central opening in the bathu, then a task should be set atop the opening to the task (Figu 156) Stern should only warm the bottom of the flaska stes should not be visible coming out nywere else in the bath. When warming fughly volatile solve the fake may need to be held on the peut of the bath to control the rate of heating (Figure 1 st or the state lowered Steam in Water Ou 49 (3....pdf Document149 (3).pdf A Document149 (2).pdf Dc Review heating and cooling techniques Minimize File Preview Page of 7 ZOOM + Bottom of the time, and steam held mot be able coming out anywhere else in the When warming highly volatile solvents, the task may need to be held above the opening of the bath to control the rate of besting (Figure 1.56e), or the serate lowered Steam in Water Out tentate ZOOM + 1.3.C TREATING MANTLES Heating mantles are a relatively safe way to heat flammable organic liquids in a round bottomed flask (Figure 1.52). The mantles are cup-shaped and designed for different sizes of round bottoned flask (Figure 1.5la). If a mantle does not fit a round bottomed flask perfectly, sand can be added to ensure good thermal coutaet (Figure 1.51c). The mantles should never be connected directly to the outlet, but first to a "Varlac" blue piece of equipment in Figure 1.516) which then connects to the outlet and delivers variable voltage to the mantie. A Varias set to "100" would be equivalent to plugging the mantle directly into the wall (100".), while a setting of"50" means the delivered voltage is halved (504). By controlling the delivered voltage. Variacs are used to regulate the temperature of a healing mantle. There is vanation between devices, and settings must be experimented with to determine appropriate beating rates (3....pdf Document149 (3).pdf Document149 (2).pdf Docume Page of 7 ZOOM + must be experimented with to determine appropriate heating rates. b Figure 1.51 Three stres of heating mantibi Hountle cenneed is a beating with and Heating mantles take some time to warm up (so may be pre-lcated during setup of an apparatus), and also take some time to cool down. The mantle will remain warm even atter turning off the Varine, and therefore flasks have to be removed from the mantle in order to cool (Figure 52c), LOWO Docum File Preview Page of 7 ZOOM + Take some time to cool down. The mantle will remain warm even after turning off the variac, and therefore flasks have to be removed from the mantle in order to cool (Figure 1.52c). b Figure 15 Distillatelede Safety note: the mam hazard wat heating mantles is that alle orme liquid spilled on the satice of a lot mantle do have the possibility ofgutien Page 5 of 7 ZOOM + 1.3.1 WATER, SAND, AND OIL BATIS Water, sand, and oil baths are related heat sources as they envelop a flask in a warm material (liquid or sand). A thermometer is often used to monitor the temperature of the bath, and is used to approximate the intemal temperature of liquid in a flask (the bath is often slightly better than the liquid in the flask), Water baths, heated on a hotplate, are most commonly used to beat solutions to 100 * (boiling baths. Figures 1.53 + 1.5ta). They may also be used to beat to lower temperatures, although it can be difficult to maintain a constant temperature. Water bathis can be covered with alumim foil to prevent excessive evaporation, or to prevent excess moisture from entering on vessels Cold water baths can also be used to cool apparahises in a quick manner (Figure 1.54b). Sand baths, also beated on a botplate, can be used to beat solutions to a wide vanety of temperatures, including very high temperatures c250 C). Sand can be placed inside Pyrex crystalling dishes (as as used with the water bath in Figure 1.50b), although Pyrex may crack it heated at too fast a nate, Sand can also be heated to moderate temperatures inside thia aluminum foil pie Fire 1.33. Bobinetter bath dishes, but should not be used at high temperatures as alummum will then oxidue, causing the thin foil to A Document 9 (3....pdf Document149 (3).pdf Document149 (2).pdf La Page 5 > of 7 ZOOM + can also be heated to moderate temperatures inside thin aluminum foil pie gure we dishes, but should not be used at high temperatures as aluminum will then oxidize, causing the thin foil to disintegrate. Thick metal pie tins (Figure 1.54c) are indestructible alternatives when high temperatures are required (but may interfere with the stirring mechanism if used), A vessel should be buried in a sand bath as much as possible as the surface is often mach cooler than the sand below. A scoopula or metal spatula can be used to pile the sand up to at least the height of liquid inside the flask Sand takes a long time to beat up, and a long time to cool down. To save time, a sand bath may be preheated while an apparatus is assembled as long as it is preheated a distance away from volatile organic liquids If the sand overheats and causes a liquid to boil controllably, the flask can be partially lifted out of the sand, or the sand moved with a metal spatula away from contact with the liquid Sand will remain warm even after turning off the hotplate, and therefore flasks have to be lifted out of the sand bath in order to cool (Ligue 1 Sid) Ilot sand baths can be cooled atop a ceramic tile b d 3....pdf Document149 (3).pdf Document14 Document149 (2).pdf heating and cooling techniques Minimize File Preview Page of 7 ZOOM + Figure 1.54: a) Herling water bb) Water bal he wildba) Allergia cool + oil baths are much like water baths, but use silicone or mineral oils in order to enable temperatures hotter than the boiling point of water (> 100 C). Silicone oil baths can be heated to 250 C. while mineral oil bxathis can be heated to 300 *c. Mineral oil is composed of mixtures of long-chain alkanes, and so is combustible. Direct contact with open flames should therefore be avoided. Oil baths can be heated in a Pyrex crystallizing dish atop a hotplate. It is also quite common for the oil to be electrically beated, through immersion of a coiled wire connected to a "Variae" (light blue piece of equipment in Figure 1.55a). A Varias connects to the outlet and can deliver variable voltage through the wire. A Variac set to "100" would be equivalent to plugging the system directly into the wall (10016). while a setting of 50" means the delivered voltage is halved (509). By controlling the delivered voltage Variaes are used to regulate the temperature of the oil. If you have previous experience using a Vanae with heating mantles, the settings will not translate to the oil bath as oil bath wires heat more rapidly than a beating mantle's wires. A paper clip can also be used in na oil bath (Figure 1550) and stirred with a stirnung plate in order to dissipate bcat. This allows for the temperature of an oil bath to quickly respond to adjustments up or down. (3....pdf Documen Document149 (3).pdf Document149 (2).pdf VA aniques Minimize File Preview Page of 7 ZOOM + CORB composition H -10.0 178 -5.3 CORTE Composition Acetone dry ice Ethyl acetate - dry ice Hexane + dry ice Methanol dry ice 36 g NaCl 100 g ice 75 g NaNO, + 100 g ice 66 g NaBr + 100 g ice Acetonitrile dry ice -84 -28 -95 -10 98 Table 1.8. Commonly used cold bathi Tee baths can be made in Tupperware containers, beakers, or almost any container (Figue 1 57a). Baths colder than -10 C should be made in an insulating container or else they cannot be easily handled and will lose beat too quickly to the room. The most common cold bath.costamer is a wide-mouthed Dewar Figure 157b), which has a vacuum jacker between the bath and the exterior An inexpensive homemade insulating hat can be made by nesting two crystalline dishes filla hep with vermiculite ta packing material) and sealing the gap with silicone caulking Ture Sex ent149 (3....pdf Document149 (3).pdf Document149 (2).pdf A Document149 28152 PHIN lating container or else they cannot be easily handled and will lose heat too quickly to the room. The most common cold bath container is a wide-mouthed Dewar (Figure 1.57b), which has a vacuum jacket between the bath and the exterior. An inexpensive homemade insulating bath can be made by nesting two crystallizing dishes, filling the gap with vermiculite (a packing material) and sealing the gap with silicone caulking (Figure 1.57e). 1.5. Collbaths bandeau Safety note: dry ice should not he handled with your beland or else you may set esthuite. Also avond direct contact with baths colder than-10 C SA Dan den Hocha Nec CHINESE Nel What is the safest way to heat ethanol ? using a bunsen burner with contents in an erlenmyer flask using a waterbath with contents in an erlenmyer flask using a hot plate with contents in a beaker using a waterbath with contents in a beaker Question 14 3 p