Question: I know its a lot but these are all the questions I am struggling with and you can take your time solving each one. I
I know its a lot but these are all the questions I am struggling with and you can take your time solving each one. I will leave a good review when you are done.
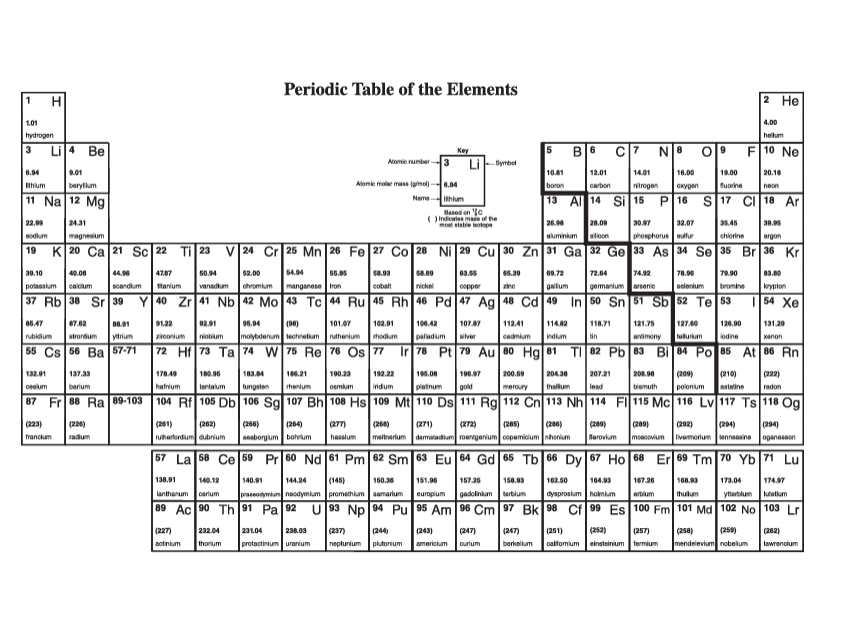
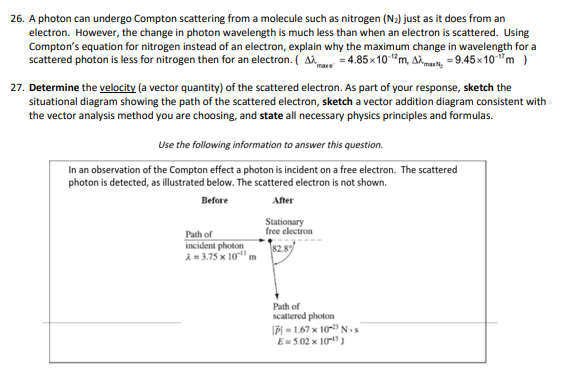

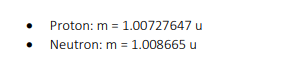
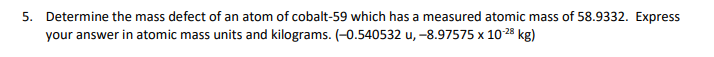





Periodic Table of the Elements H 2 He 4.DD hydrogen 3 Li 4 Be Key B 6 C 7 N B O 19 F 10 Ne Atomic runbar- 3 Li --Symbol 8.94 1.01 10.61 12.01 14.01 18.05 18.00 20.18 thium barylium Alani molar mass (pinol) -= 6.94 carbo nitrogen ikyper noon 11 Na 12 Mg ichlum 13 Al 14 Si 15 P 16 $ 17 C 18 Ar M.31 32.07 sodium magnesium aluminium ailloon phosphorus ulu chiering argon 19 K 20 Ca 21 Sc 22 Ti 23 V 24 Cr 25 Mn 26 Fe 27 Co 28 Ni 20 Cu 30 Zn 31 Ga 32 Ge 33 As 34 Se 35 Br|36 Kr 39,10 D.M 62.00 6404 09.72 72.64 potassium Itanium vanadium chromium manganese Iron Dbal nickel copper ring gallium gormanium arsenic arionium aypion 37 Rb 38 Sr 39 40 Zr 41 Nb 42 Mo 43 To 44 Ru 45 Rh 46 Pd 47 Ag 48 Cd 49 In 50 51 Sb 52 Te 53 54 Xe 15,47 15.04 101 87 02.01 180.42 107 47 112.4 11483 118.71 121.75 120.90 131 30 strontium yirium non um molybdenum nathanlum thedium palladium addmium antimony lodina 55 Cs 56 Ba 57-71 72 Hf 73 Ta 74 W 75 Re 76 OS 77 Ir 78 Pt 79 Au 80 Hg 6 83 Bi$4 Po 85 At 86 An 132.01 137.35 178.49 189.96 185.84 186.21 182.2 185.08 190 87 200.59 204.50 207.21 208.08 (209) (218) (232) barium hatrium aninlum tungale osmium idium Platinum heroury hallum polonium mining radon 87 Fr 88 Ra 89-103 104 Rf 105 Db 106 Sq 107 Bh 108 Hs 109 Mt 110 Ds 111 Rg 112 Cn 113 Nh 114 F| 115 Mc 116 Ly 117 Ts 118 Og (323 1224) 262 264 1271) (272 (285) 246 2891 (202) (204) francium radium nutharderdum cubrium inaborg tim bohrum hasslum molinarium roar garlum copam cum opanassen 57 La 58 Ce 59 Pr 60 Nd 61 Pm 62 Sm 63 1 64 Gd 65 Th 66 DV 67 Ho 68 Er 69 Tm Yb 71 Lu 138.91 140. 144.94 150.10 151.96 157 35 150.03 162.50 164.03 167.20 173.04 lanthanum carlum paseodymium hoodyinlum promethium samarium ouropium gadol nium Barblum dysposium holmlun arb um thulu yborblum lutallum 89 Ac Th 91 Pa 92 U 93 Np 94 Pu 95 Am 96 Cm 97 BK 98 Cf | 99 Es 100 Fm 101 Ma 102 No 103 Lr (227) 232.0 231.0 286.09 (244) (243) 1247 (247) (251] (2571 (258 (262) solinium protactinium uranium neptunium plutonium amaricium wrium berkellum caltomium instein um lermium mendelevium nobellum awrenoium26. A photon can undergo Compton scattering from a molecule such as nitrogen (Na) just as it does from an electron. However, the change in photon wavelength is much less than when an electron is scattered. Using Compton's equation for nitrogen instead of an electron, explain why the maximum change in wavelength for a scattered photon is less for nitrogen then for an electron. ( A) = 4.85x10""m, A).. =9.45x10-"m } 27. Determine the velocity (a vector quantity) of the scattered electron. As part of your response, sketch the situational diagram showing the path of the scattered electron, sketch a vector addition diagram consistent with the vector analysis method you are choosing, and state all necessary physics principles and formulas. Use the following information to answer this question. In an observation of the Compton effect a photon is incident on a free electron. The scattered photon is detected, as illustrated below. The scattered electron is not shown. Before After Stationary Path of free electron incident photon A = 3.75 x 10 m Path of scattered photon [7) = 1.67 x 10- N . S E- 5.02 x 105 ]Lesson 2: Millikan's Oil Drop Experiment 9. A charged particle with a mass of 40 g is suspended in a Millikan oil drop apparatus that has a separation of 4.0 cm between the charged plates. If the potential difference required for the suspension is 1.633 x 101 V, what is the number of excess electrons on the charged particle? (6)\f5. Determine the mass defect of an atom of cobalt-59 which has a measured atomic mass of 533332. Express your answer in atomic mass units and kilograms. {-0.54532 u; -3.E?5T5 x 10'13 kg} E. For radium226 {atomic mass = 225E254 u} obtain {a} the mass defect, [b] the binding energy in Me'u', and it] the binding energy per nucleon. [-1.310653 u,-1E-91.25 Me'u', 4.4334 Mewnucleen] 17. Find the energy in MeV released when alpha decay converts thorium-228 (228.028715 u) into radium-224 (224.020186 u). The mass of an alpha particle is 4.002603 u. (-5.54 MeV)Lesson 3: Fission and Fusion 18. Given the following atomic masses, [answer = 2.93 x 10-1] ]] 235 U = 3.9029x10 - kg n =1.6749x10-27 kg 140 Xe = 2.3234x10- kg 94 Sr =1.5595x10-25 kg determine the energy produced in the following fission reaction: OU+ on - `54Xe + 94Sr 38 Sr + 2an 19. Given the following masses, [answer = 2.83 x 10-12 ]] "H=3.3444x10- kg 3H=5.0082x10-2 kg n =1.6749x10-2 kg #He = 6.6463x10- kg determine the energy produced in the following fusion reaction: H + H - He+ on 20. Calculate the energy released by the reaction. [answer = 172.9 Mev] Te+ 3,n Masses: uranium-235 = 235.043930, n = 1.008665, zirconium-94 = 93.906315, tellurium-139 = 138.934700 1 u = 1.660539 x 10-27 kg29. The mass of 3 psi particle is EDS? GEWCI. Express this mass in kilograms. [5.5115 1; III\" kg] 31. In the B* decay of nitrogen-12 (12.01864 u) into carbon-12 (12.00000 u), a positron with energy of 11.0 MeV is emitted. What is the energy of the electron neutrino? Assume the mass of the positron is 5.485799 x 10* u (same as an electron) (5.8 Mev)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


