Answered step by step
Verified Expert Solution
Question
1 Approved Answer
i need a quick answer A compound has the following percent composition. C. 40% H:6,67%, 0:53 3%. Its molecular weight is 60. Derive its molecular
i need a quick answer 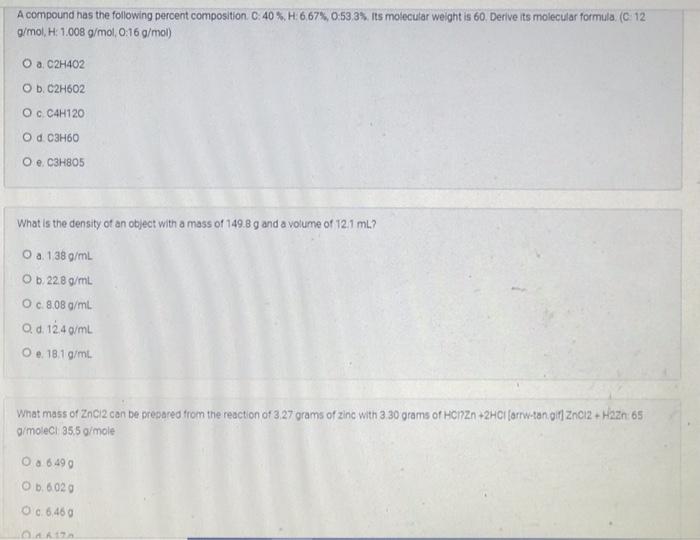
A compound has the following percent composition. C. 40% H:6,67%, 0:53 3%. Its molecular weight is 60. Derive its molecular formula. (C 12 g/mol, H: 1.008 g/mol,0:16 g/mol) O a C2H402 O b. C2H602 Oc C4H120 O d. C3H60 O e C3H805 What is the density of an object with a mass of 149.8 g and a volume of 121 ml? O a. 138 g/L Ob 22.8 g/mL O c. 8.08 g/mL O d. 12.4 g/mL O.. 18.19/mL. What mass of 2ncl2 can be prepared from the reaction of 3 27 grams of zinc with 3 30 grams of HonZn +2HCl (arrw-tan alt ZnCl2 + Haze: 65 g/moleci 355 g/mole 0.8 6 490 O b. 6020 0.6.460 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


