Answered step by step
Verified Expert Solution
Question
1 Approved Answer
i need answer plz 3. Consider the following reaction equation: [Pt(dien)C1=+H:0 [Pl(dien)(H:0)P+CIS The plot Ink/T) versus 1/T-is-a-line with intercept:15.89 anda slope -10001.37.1 a) Calculate AH
i need answer plz 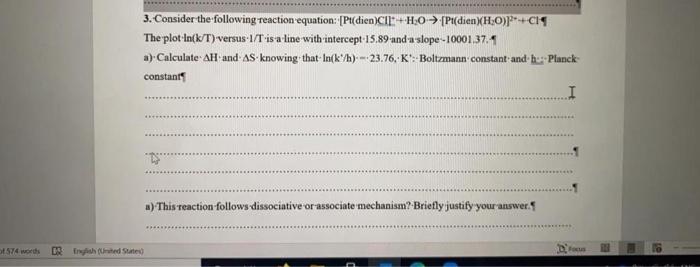
3. Consider the following reaction equation: [Pt(dien)C1=+H:0 [Pl(dien)(H:0)P+CIS The plot Ink/T) versus 1/T-is-a-line with intercept:15.89 anda slope -10001.37.1 a) Calculate AH and AS knowing that In(k/h) --23.76,-K'. Boltzmann constant and be: Planck constants a) This reaction follows dissociative or associate mechanism? Briefly justify your answer. ST4 words Raph (ted States) Focus 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


