Answered step by step
Verified Expert Solution
Question
1 Approved Answer
i need help calculating the last 3 boxes Gas Stoichiometry Background You have just been hired as an intern at Chemists R Wii Institute, a
i need help calculating the last 3 boxes 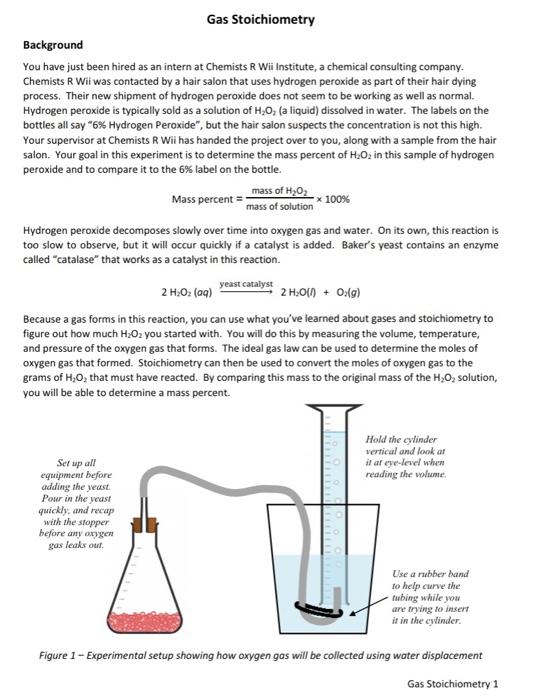
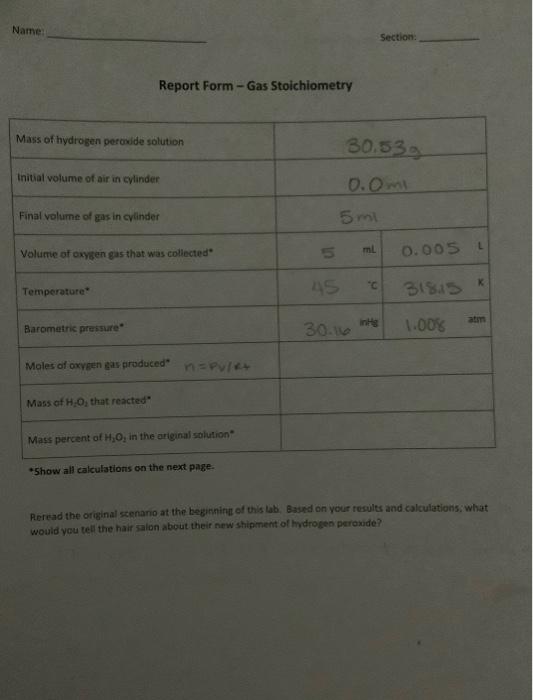
Gas Stoichiometry Background You have just been hired as an intern at Chemists R Wii Institute, a chemical consulting company. Chemists R Wii was contacted by a hair salon that uses hydrogen peroxide as part of their hair dying process. Their new shipment of hydrogen peroxide does not seem to be working as well as normal. Hydrogen peroxide is typically sold as a solution of H,0, (a liquid) dissolved in water. The labels on the bottles all say "6% Hydrogen peroxide", but the hair salon suspects the concentration is not this high. Your supervisor at Chemists R Wii has handed the project over to you, along with a sample from the hair salon. Your goal in this experiment is to determine the mass percent of H.Oz in this sample of hydrogen peroxide and to compare it to the 6% label on the bottle. Mass percent mass of H202 mass of solution 100% Hydrogen peroxide decomposes slowly over time into oxygen gas and water. On its own, this reaction is too slow to observe, but it will occur quickly if a catalyst is added. Baker's yeast contains an enzyme called "catalase" that works as a catalyst in this reaction. yeast catalyst 2 H2O2 (aq) 2 H:01h + O2(g) Because a gas forms in this reaction, you can use what you've learned about gases and stoichiometry to figure out how much H2O2 you started with. You will do this by measuring the volume, temperature, and pressure of the oxygen gas that forms. The ideal gas law can be used to determine the moles of oxygen gas that formed. Stoichiometry can then be used to convert the moles of oxygen gas to the grams of HO, that must have reacted. By comparing this mass to the original mass of the H2O2 solution, you will be able to determine a mass percent. Hold the cylinder vertical and look ar it al eye-level when reading the volume Set up all equipment before adding the yeast Pour in the yeast quickly, and recap with the stopper before any oxygen gas leaks out. Use a rubber band to help curve the tubing while you are trying to insert it in the cylinder Figure 1 - Experimental setup showing how oxygen gas will be collected using water displacement Gas Stoichiometry 1 Name Section: Report Form - Gas Stoichiometry Mass of hydrogen peroxide solution 30.53 Initial volume of air in cylinder 0.0 m 5ml Final volume of gas in cylinder Volume of oxygen gas that was collected" 5 ml 0.005 L K Temperature 45 31815 atm Barometric pressure 30.116 1.00% Moles of oxygen gas produced Mass of H.O, that reacted" Mass percent of 1,0, the original solution *Show all calculations on the next page. Reread the original scenano at the beginning of this lab. Based on your results and calculations, what would you tell the hair salon about their new shipment of hydrogen peroxide 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


