Question
I need help calculating the specific heat of two unknown metal samples and identifying which metal they are in my general chemistry I calorimetry lab.
I need help calculating the specific heat of two unknown metal samples and identifying which metal they are in my general chemistry I calorimetry lab.
Below is the equation needed to use: NOTE- specific heat of water is 4.18
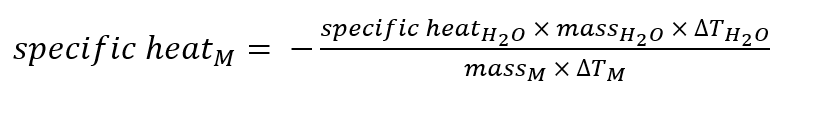
Below are the data from my two trials, we were heating the metal in a test tube and that test tube was in a beaker of water, while preparing the calorimeter with 20ml of room temp. water

initial temperature of metal: 21.00 degrees C
Trial 2 data:
mass of unknown metal: 20.3852g
calorimeter (cups and lid) mass: 10.7164g
20mL H2O+calorimeter mass: 29.4955g
temperature of metal after 10 minutes of heating: 99.2 degrees C
temperature of water after 10 minutes of heating: 99.2 degrees C
temperature of water in calorimeter: 20.2 degrees C
initial temperature of metal: 20.9 degrees C
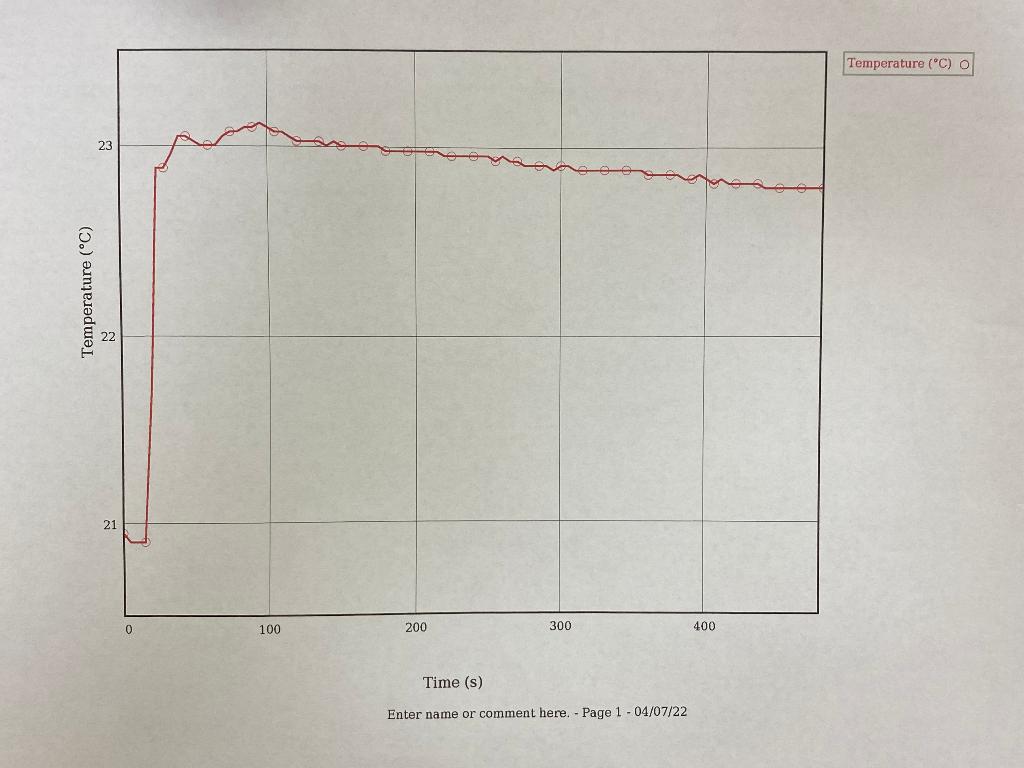
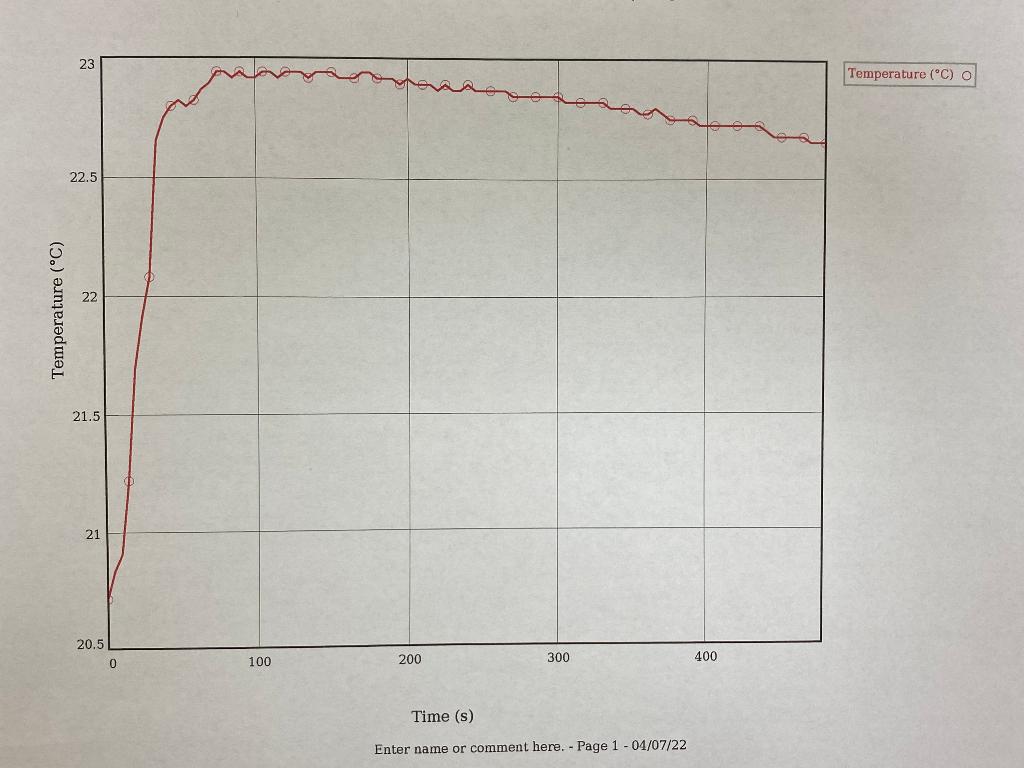
After we heated the metal and prepared our calorimeter, we added the metal into our calorimeter with 20mL of water and recorded the heat over time, this is shown in the two graphs below. (I think this is where delta T would be obtained)
TRIAL 1 GRAPH:
TRIAL 2 GRAPH:
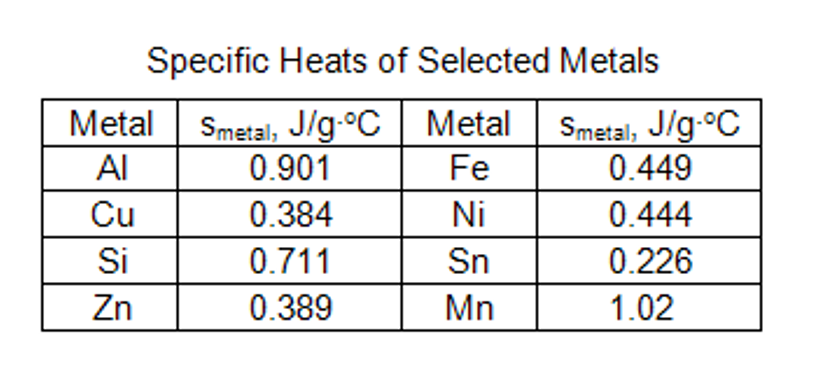
After you've found the specific heat capacity for both trials, we need to identify which metal trials 1 and 2 are, using the table below. (I used the same metal sample for trial 1 and 2) so only one metal should be identified.
please show work, thank you.
specific heat = specific heath20 X massh20 X AT H20 masSM X Data collection and observations -Thalt: Mass of unknown metali 16.5933 ig Calorimeter (cups and lid) mass: 10.2491 g 20 mL HO + Calorimeter mass: 29.5761 g Temperature of metal after 10 min. of heating? Temperature of water after lo min. of heating Temperature of water in calorimeter: 20.9C D 98.5 C 98.5 C Temperature (C) O Temperature (C) 21 0 100 200 300 400 Time (s) Enter name or comment here. - Page 1 - 04/07/22 23 Temperature (C) O 22.5 Temperature (C) 21.5 21 20.5 0 100 200 300 400 Time (s) Enter name or comment here. - Page 1 - 04/07/22 Specific Heats of Selected Metals Metal Smetal, J/g C Metal Smetal, J/g.C 0.901 Fe 0.449 Cu 0.384 Ni 0.444 Si 0.711 Sn 0.226 Zn 0.389 Mn 1.02Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


