Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I need help figuring out how my professor solved for T. I'm having trouble seeing how they removed the T from the exponent. (McQuarrie 17-43)
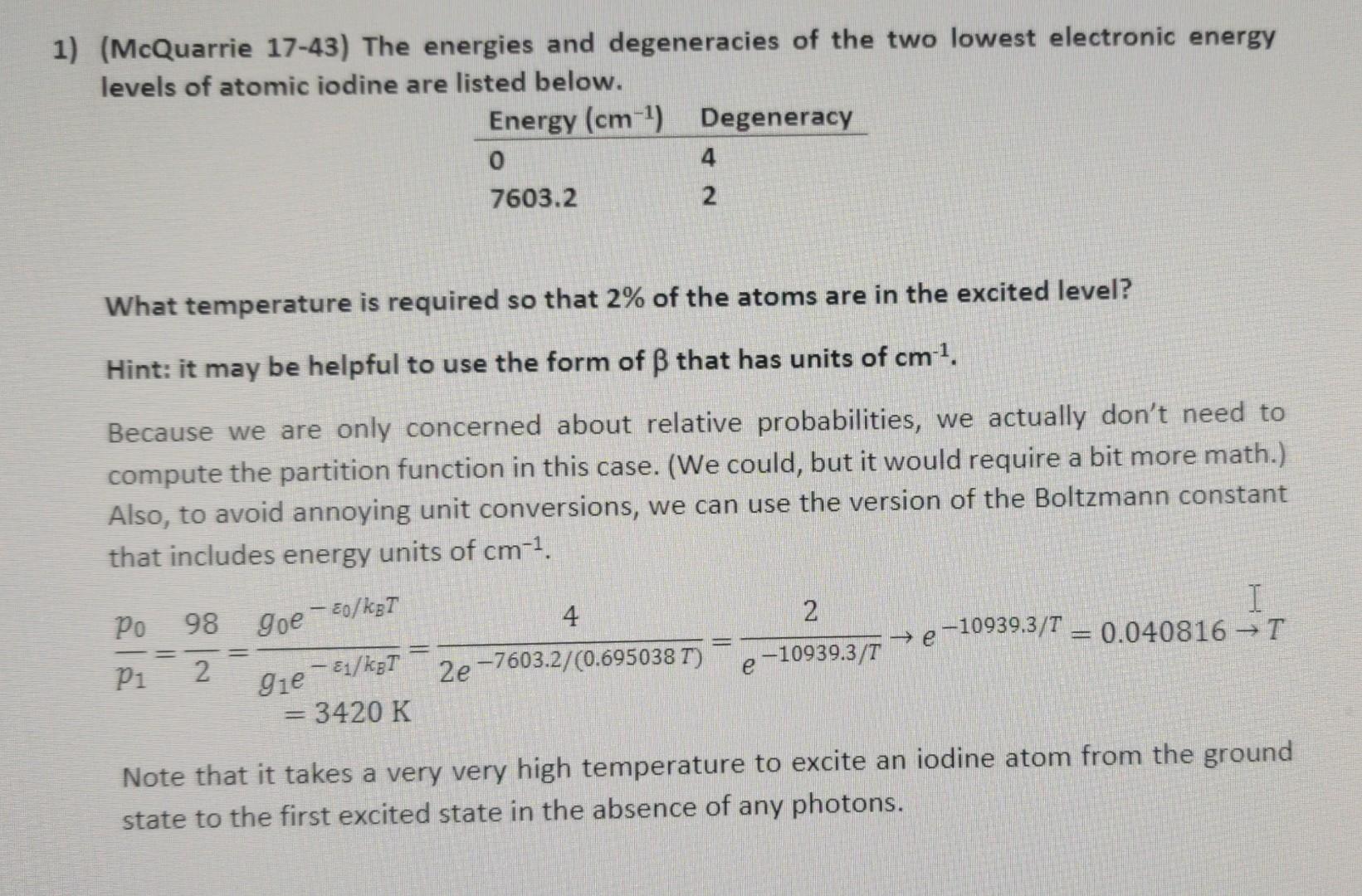
I need help figuring out how my professor solved for T. I'm having trouble seeing how they removed the T from the exponent.
(McQuarrie 17-43) The energies and degeneracies of the two lowest electronic energy levels of atomic iodine are listed below. What temperature is required so that 2% of the atoms are in the excited level? Hint: it may be helpful to use the form of that has units of cm1. Because we are only concerned about relative probabilities, we actually don't need to compute the partition function in this case. (We could, but it would require a bit more math.) Also, to avoid annoying unit conversions, we can use the version of the Boltzmann constant that includes energy units of cm1. p1p0=298=g1e1/kBT=3420Kg0e0/kBT=2e7603.2/(0.695038T)4=e10939.3/T2e10939.3/T=0.040816T Note that it takes a very very high temperature to excite an iodine atom from the ground state to the first excited state in the absence of any photonsStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


